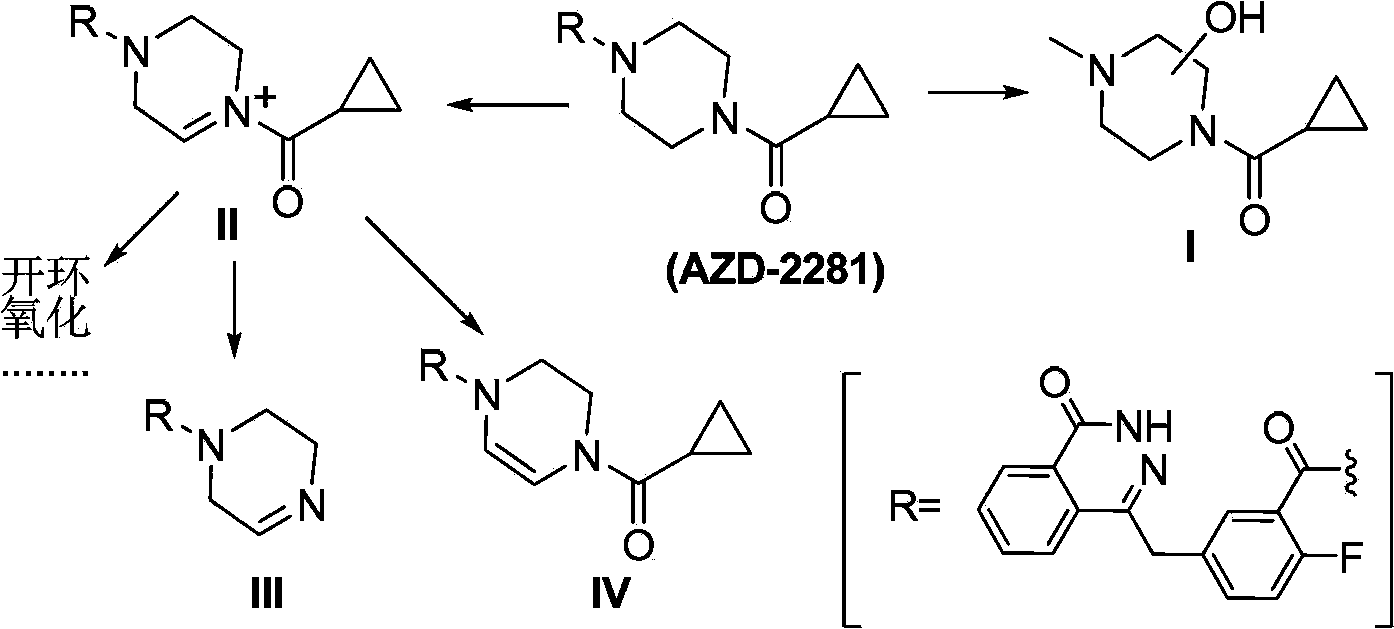
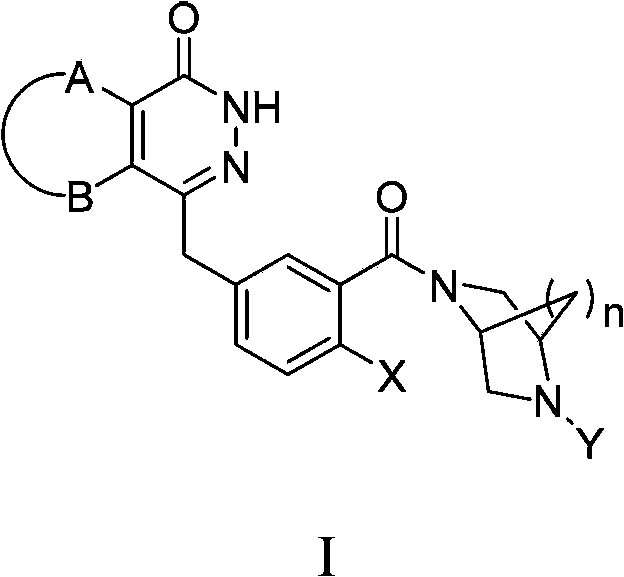
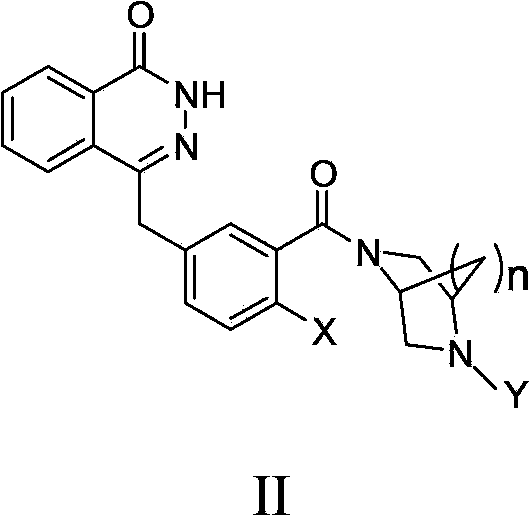
Condensed ring pyridazinone compound with bridge ring structure as well as preparation method and application thereof
A bridged ring structure and compound technology, applied in medical preparations containing active ingredients, organic chemistry, drug combinations, etc., can solve problems such as difficult research and development, unstable metabolism, and low bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0091] The present invention will be further described below in conjunction with specific examples, but these examples do not limit the scope of the present invention.
[0092] One, preparation embodiment
[0093] 1 H-NMR was measured with a Varian Mercury AMX300 instrument; MS was measured with a VG ZAB-HS or VG-7070 instrument, and all were EI sources (70ev) unless otherwise noted; all solvents were re-distilled before use, and no The water solvents were all obtained by drying according to standard methods; except for the instructions, all reactions were carried out under nitrogen protection and followed by TLC, and the post-treatment was washed with saturated sodium chloride aqueous solution and dried with anhydrous sodium sulfate; the purification of the product Unless otherwise specified, silica gel (200-300 mesh) column chromatography was used; among them, silica gel (200-300 mesh) was produced by Qingdao Ocean Chemical Factory, and GF254 thin-layer silica gel plate was...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com