SiRNA (Small interfering Ribonucleic Acid)/anti-cancer drug combined transferring composite supporter and preparation method and application thereof
A composite carrier, anticancer drug technology, applied in drug combinations, antitumor drugs, pharmaceutical formulations, etc., can solve the problems of low transfection efficiency of non-viral vectors, easy to induce immune response, large particles in the delivery system, etc. Cytotoxicity, achieving targeting, increasing the effect of loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
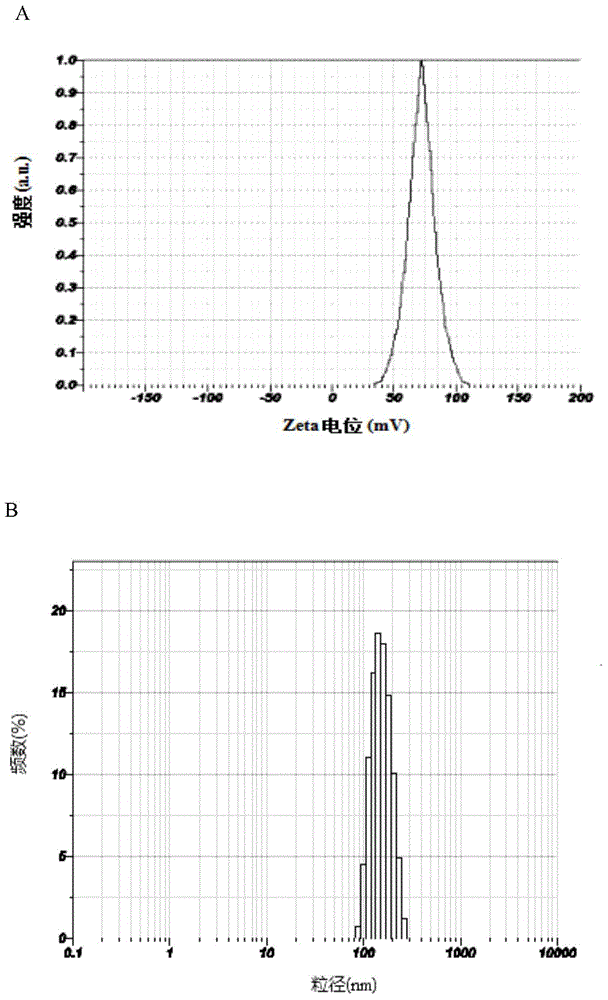
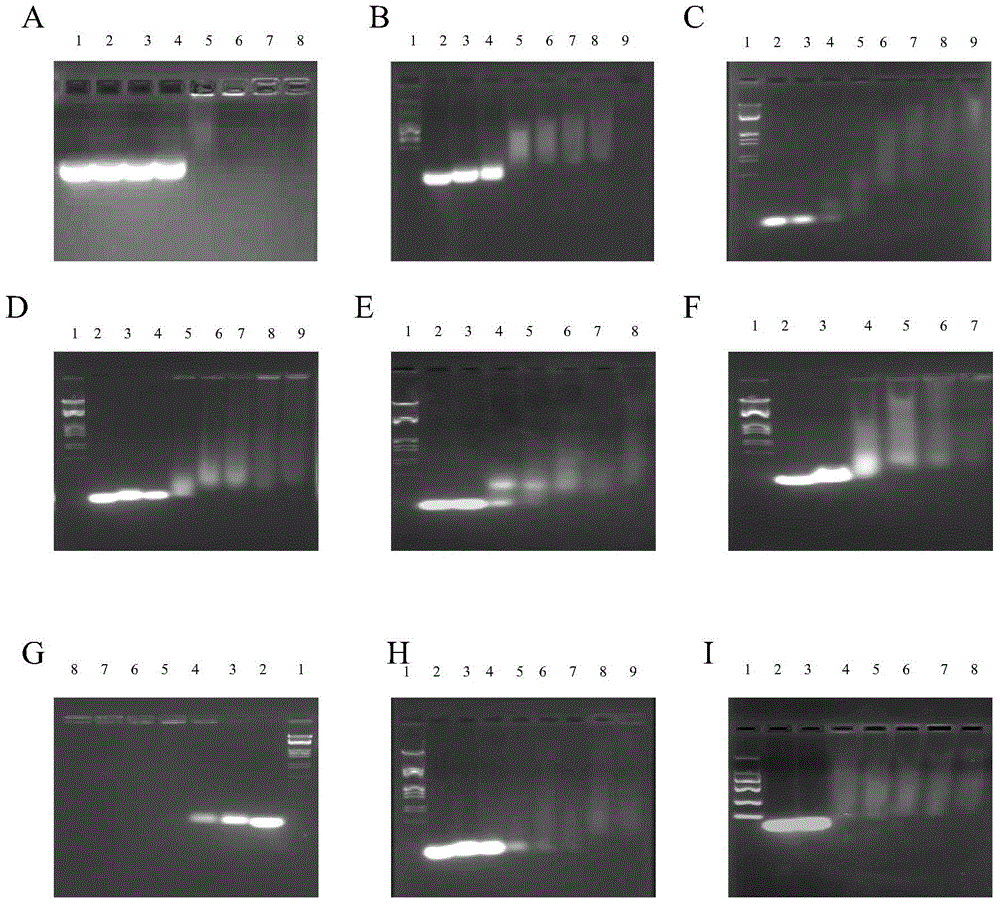
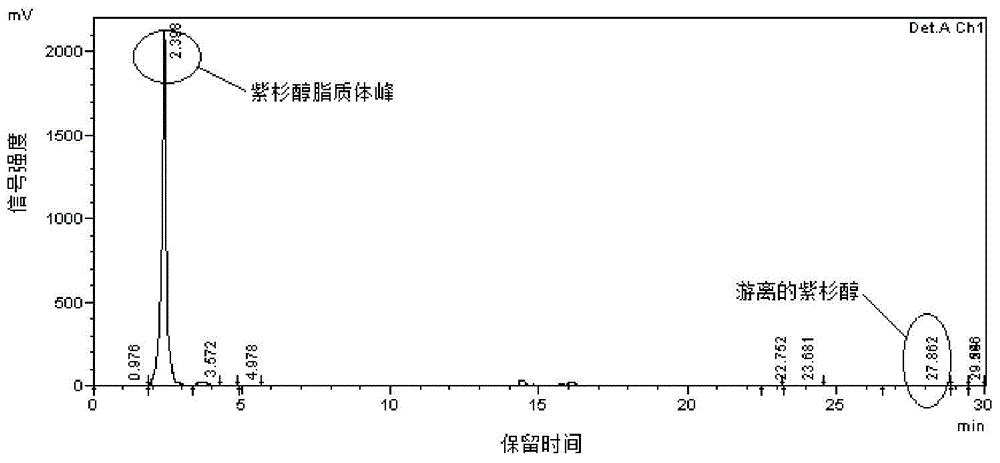
Image
Examples
Embodiment 1
[0090] Weigh a certain mass of new-type cationic lipid DDCTMA, dissolve DDCTMA and paclitaxel in 1ml of chloroform at a mass ratio of 200 / 1, after fully dissolving, blow the film with nitrogen, dry in vacuum overnight, add 1ml of ultrapure water at 55°C and place in The paclitaxel liposome (DDCTMA-TAX200 / 1) was prepared in a water bath at 55°C for 2-3 hours, and repeatedly ultrasonically oscillated at 55°C until clarified.
[0091] Compression of siRNA: Sulfate protamine (belonging to cationic polymer) is dissolved in 5% glucose aqueous solution, siRNA and hyaluronic acid (belonging to glycosaminoglycan) are mixed at a mass ratio of 1:1, and the above mixed liquid droplets Add it into protamine sulfate solution, the mass ratio of protamine sulfate to siRNA is 5:1, vortex and oscillate to mix, and incubate at room temperature for 20 minutes to obtain compressed siRNA for later use.
[0092] The paclitaxel liposome and the prepared compressed siRNA were mixed at a mass ratio of ...
Embodiment 2
[0095] Weigh a certain amount of new lipid DDCTMA, and dissolve DDCTMA and paclitaxel in 1ml of chloroform at a mass ratio of 200 / 1. After fully dissolving, add DOPE with a molar ratio of 1 / 1 to DDCTMA, blow film with nitrogen, and vacuum dry Overnight, add 1 ml of ultrapure water at 55°C and place in a water bath at 55°C for 2-3 hours, and repeatedly ultrasonically shake at 55°C until clarified to prepare paclitaxel liposomes (DDCTMA-TAX+D200 / 1).
[0096] Compression of siRNA: Protamine sulfate was dissolved in 5% glucose aqueous solution, protamine sulfate and siRNA were mixed at a mass ratio of 4:1, vortexed and oscillated, incubated at room temperature for 20 minutes, and compressed siRNA was obtained for use.
[0097] Mix paclitaxel liposomes with the prepared compressed siRNA at a mass ratio of 1 / 1, 2 / 1, 3 / 1, 4 / 1, 5 / 1, and 10 / 1, vortex slightly, and incubate at room temperature for 15-20 minutes to obtain Modified composite carrier.
[0098] The composite carrier prepar...
Embodiment 3
[0100] Weigh a certain amount of new lipid DDCTMA, and dissolve DDCTMA and paclitaxel in 1ml of chloroform at a mass ratio of 100 / 1. After fully dissolving, blow the film with nitrogen and dry it in vacuum overnight. °C for 2-3 hours in a water bath, repeated ultrasonic vibration at 55 °C until clarified, and paclitaxel liposomes (DDCTMA-TAX100 / 1) were prepared.
[0101] Compression of siRNA: Dissolve protamine sulfate in 5% glucose aqueous solution, mix siRNA and hyaluronic acid at a mass ratio of 1:1, add the above mixture dropwise to protamine sulfate solution, protamine sulfate The mass ratio of siRNA to siRNA was 2:1, vortexed to mix, and incubated at room temperature for 20 min to obtain compressed siRNA for later use.
[0102] Mix paclitaxel liposomes with the prepared compressed siRNA at a mass ratio of 1 / 1, 4 / 1, 8 / 1, 12 / 1, 16 / 1, and 20 / 1, vortex slightly, and incubate at room temperature for 15-20 minutes to obtain Modified composite carrier.
[0103] The composite ...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap