Protein-free coated plate confining liquid
A technology of coating plate and sealing liquid, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., to achieve the effects of short sealing time, stable properties and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] The procedure of the hepatitis C core antigen detection kit (ELISA) established with the hepatitis C core antigen (HCV) monoclonal antibody coated in the embodiment of the present invention is the same as that of the comparative example 1, the difference is that in step 1, the amount of blocking solution coated on the plate is The components are:
[0065] Tris-HCl buffer solution (pH=7.4) 0.1mol / L,
[0066] PEG-20000 1g / L,
[0067] Tween-20 (Tween-20) 5g / L,
[0068] Trehalose 10g / L,
[0069] Sodium azide 1g / L.
[0070] Discard the coating solution in the coated plate, pat the coated plate dry, add the above-prepared coated plate blocking solution at 100 μL to 300 μL / well, and coat at 37°C for 2 hours; the rest of the operations are carried out as compared The operation is the same as in example 1. The test results are shown in Table 2 below.
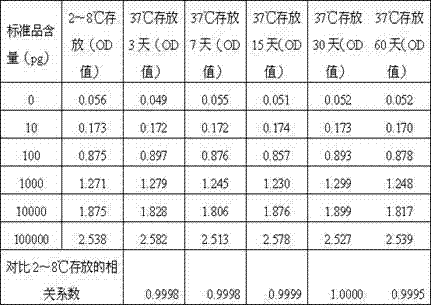
[0071] Table 2 The OD values of standard substances with different concentrations detected under different storage condi...
Embodiment 3
[0091] Table 4 Comparative Example 3 Detection Results
[0092]
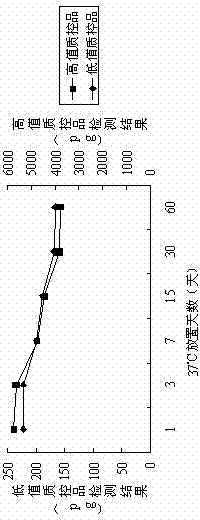
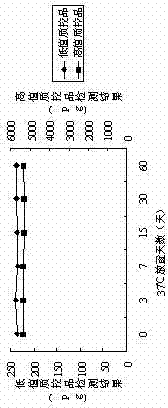
[0093] Through the experimental data, it can be seen that the coated plates prepared in Example 1 and Comparative Examples 1, 2, and 3: in the absence of BSA blocking, the reagent blank absorbance will be relatively low; In the case of sugar, the prepared coated plate can be stored stably at 37°C for 60 days without damage, and the correlation coefficient is still above 0.99. If macromolecular substances or trehalose are added alone, the above requirements cannot be met.
Embodiment 2
[0095] The steps of the hepatitis C core antigen detection kit (ELISA) established with the hepatitis C core antigen (HCV) monoclonal antibody coated in the embodiment of the present invention are the same as in Example 1, except that the composition of the blocking solution for the coated plate in step 1 Divided into:
[0096] Tris-HCl buffer solution (pH=7.4) 0.01mol / L,
[0097] Polyvinylpyrrolidone 0.5g / L,
[0098] Tween-20 (Tween-20) 0.5g / L,
[0099] Trehalose 1g / L,
[0100] Sodium azide 0.1g / L.
[0101] The experimental operation flow is the same as that described in Example 1.
[0102] Example 3
[0103] The steps of the hepatitis C core antigen detection kit (ELISA) established with the hepatitis C core antigen (HCV) monoclonal antibody coated in the embodiment of the present invention are the same as in Example 1, except that the composition of the blocking solution for the coated plate in step 1 Divided into:
[0104] Tris-HCl buffer solution (pH=7.4) 0.05mol / L...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com