Method of stable fluorescence labeling of living cells of oral squamous cell carcinoma
A technique of oral squamous cell carcinoma and fluorescent labeling, which is used in measurement devices, instruments, disease diagnosis, etc., can solve the problem of inability to label the living cells of oral squamous carcinoma cells, and achieves the effects of stable imaging, long storage time and high fluorescence intensity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) According to the product instructions, the near-infrared fluorescent material Alexa680 (Invitrogen Company) was coupled with Nimotuzumab monoclonal antibody (Baitai Company) to prepare the fluorescently labeled Nimotuzumab monoclonal antibody probe (Alexa680-Nim);
[0032] (2) Digest oral squamous cell carcinoma CAL27 cells in the logarithmic growth phase, count them under an inverted phase-contrast microscope, centrifuge and discard the supernatant, and add PBS to adjust the cell density to 2×10 6 / mL, and then divide the cell suspension into dedicated flow tubes, 100 μL / tube.
[0033] (3) Add Alexa680-Nim to a final concentration of 25 μg / mL in PBS, and incubate at 4°C in the dark for half an hour.
[0034] (3) Labeling experiment: divided into experimental group and control group
[0035] Experimental group: add Alexa680-Nim to make the final concentration in PBS 25 μg / mL, and incubate at 4°C in the dark for half an hour;
[0036] Control group: including the f...
Embodiment 2
[0044] (1) According to the product instructions, the near-infrared fluorescent material Alexa680 was coupled with Nimotuzumab monoclonal antibody to prepare the fluorescently labeled Nimotuzumab monoclonal antibody probe (Alexa680-Nim);
[0045] (2) Well-grown CAL27 cells were digested and passaged in a glass-bottom culture dish with a diameter of 35 mm and a central glass-bottom aperture of 10 mm. After 2-3 days, the cells grew to 70-80%.
[0046] (3) 12 hours before labeling, replace the cell culture medium to be used with serum-free medium, gently aspirate the medium before labeling, and wash twice with PBS.
[0047] (4) Add 50 uL of serum-free medium containing Alexa680-Nim to the central glass-bottom well at a concentration of 100 μg / mL, and incubate at 4 °C in the dark for half an hour.
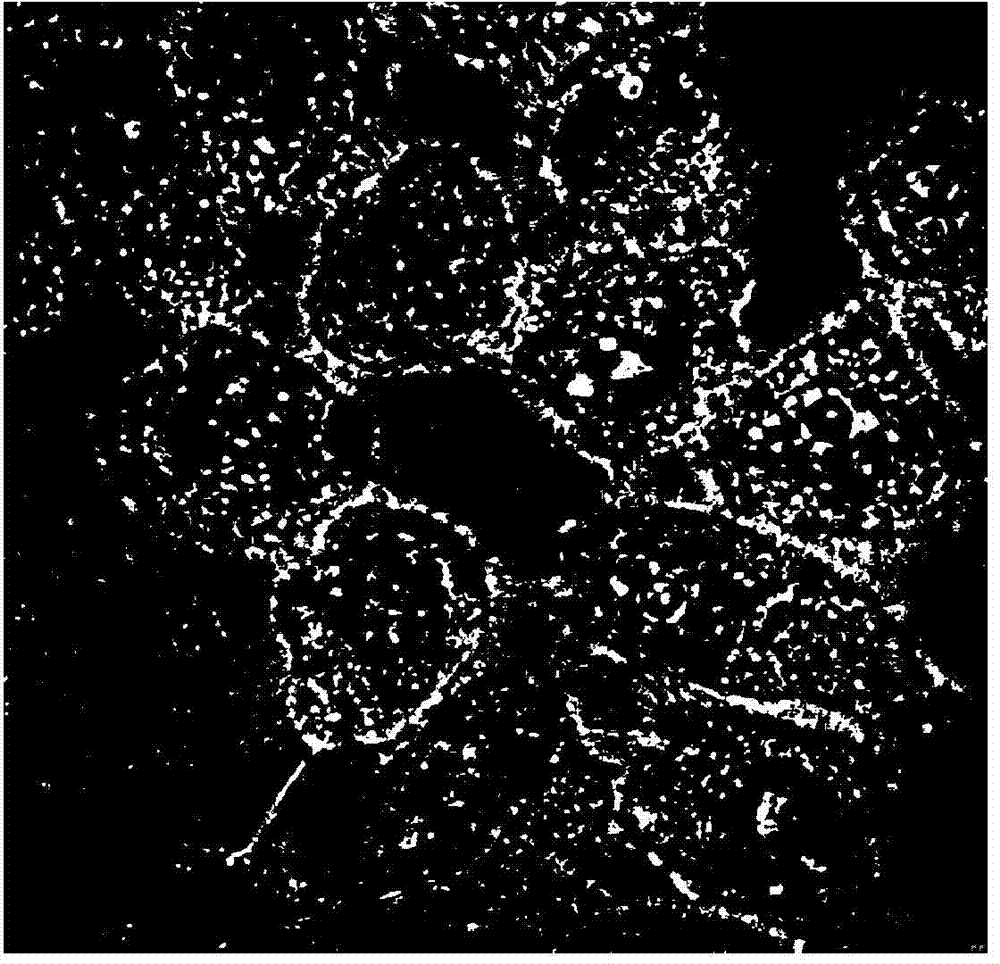
[0048] (5) Take out the cells, suck off the liquid, wash with PBS 3 times, add a small amount of PBS, and observe under a confocal microscope.
[0049] (6) Confocal microscope observa...
Embodiment 3
[0051] (1) According to the product instructions, the quantum dot Qdot800 (Invitrogen Company) was coupled with the Nimotuzumab monoclonal antibody (Baitai Company) to prepare the fluorescent quantum dot Nimotuzumab monoclonal antibody probe (Qdot800-Nim);
[0052] (2) Digest oral squamous cell carcinoma CAL27 cells in the logarithmic growth phase, count them under an inverted phase-contrast microscope, centrifuge and discard the supernatant, and add PBS to adjust the cell density to 2×10 6 / mL, then divide the cell suspension into special flow tubes, 100 μL / tube, add different amounts of experimental reagents to the flow tubes according to the following design;
[0053] (3) Labeling experiment: divided into experimental group and control group
[0054] Experimental group: add Qdot800-Nim to a final concentration of 20nmol / L in PBS, and incubate at 4°C in the dark for half an hour;
[0055] Control group: including the following four groups, and the rest of the treatment and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Excitation wavelength | aaaaa | aaaaa |
| Excitation wavelength | aaaaa | aaaaa |
| Excitation wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com