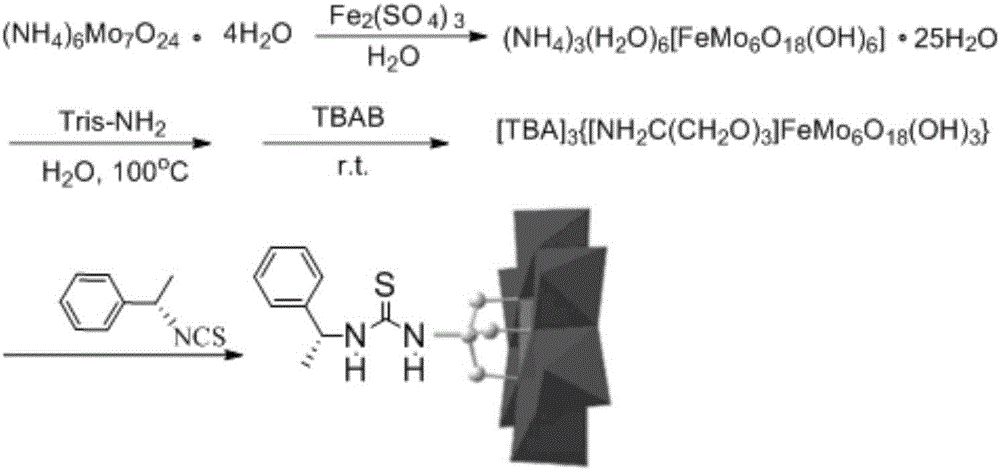
(S)-1-(1-phenethyl)thiourea unilateral-modified Fe-Anderson-type heteropoly acid catalyst, preparation method and application thereof
A technology of heteropolyacid and phenethyl group, which is applied in the field of Fe-Anderson type heteropolyacid catalyst preparation, can solve the problems of recycling and reusing expensive catalysts, and achieve the effect of solving expensive catalysts, mild reaction conditions and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Fe-Anderson type heteropolyacid precursor (NH4 ) 3 (H 2 O) 6 [FeMo 6 o 18 (OH) 6 ]·25H 2 Preparation of O
[0031] Take 15.9g (12.9mmol) ammonium molybdate and add it to 250ml deionized water, stir well to obtain a transparent liquid, then add concentrated hydrochloric acid dropwise to adjust the pH of the system to 2-4. Heat to boiling, slowly add 3.2g (9.3mmol) ferric sulfate solid, stir for 1h, a small amount of solid is produced, stop stirring, and suction filter while it is hot to obtain a brownish-red liquid, place it at room temperature, let it stand for 48h, recrystallize once, A white solid (NH 4 ) 3 (H 2 O) 6 [FeMo 6 o 18 (OH) 6 ]·25H 2 O, the yield is 80.3%.
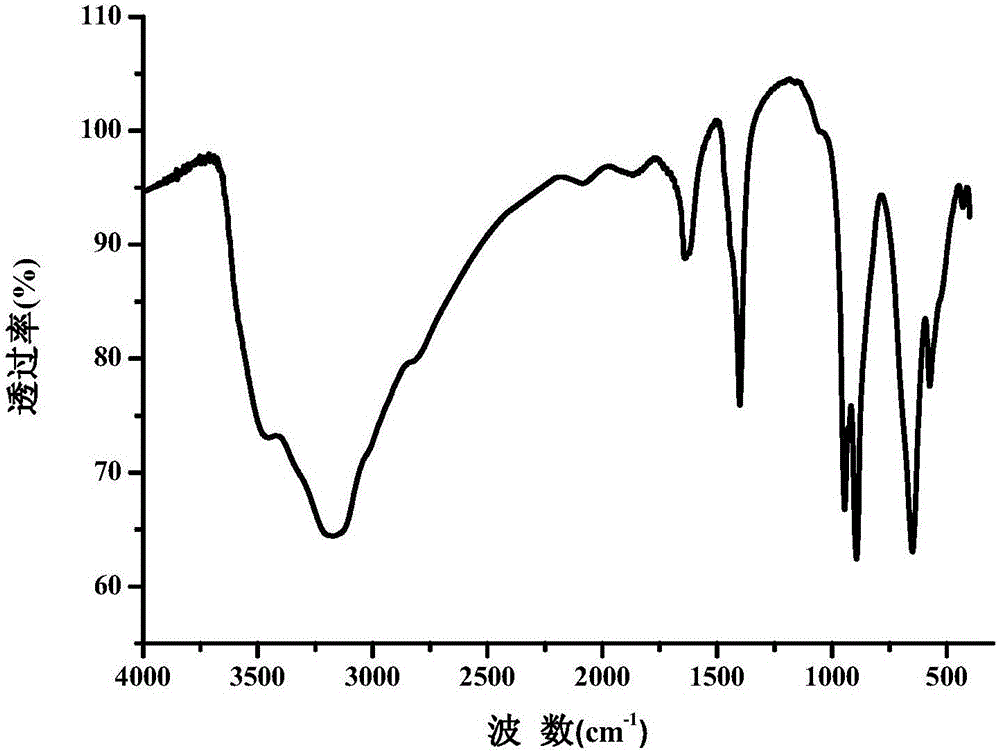
[0032] Parent (NH 4 ) 3 (H 2 O) 6 [FeMo 6 o 18 (OH) 6 ]·25H 2 The infrared spectrum of O is as figure 2 shown.
Embodiment 2
[0034] Preparation of (S)-1-(1-phenylethyl)isothiocyanate
[0035] Add (S)-(+)-1-phenylethylamine (0.606g, 5mmol) into a dry reaction vessel, dissolve it with 20mL of alcohol, then slowly add CS 2 (0.1142g, 15mmol) and triethylamine (0.506mg, 5mmol), after stirring the reaction at room temperature for 1h, then adding di-tert-butyl dicarbonate (Boc 2 O) (1.091mg, 5mmol) and 4-dimethylaminopyridine (DMAP) (18mg, 0.15mmol), after stirring and reacting at room temperature for 2h (gas is generated during the stirring process, attention should be paid to degassing and decompression), which can be obtained 0.7451 g (S)-1-(1-phenylethyl)isothiocyanate. The yield was 91.3%.
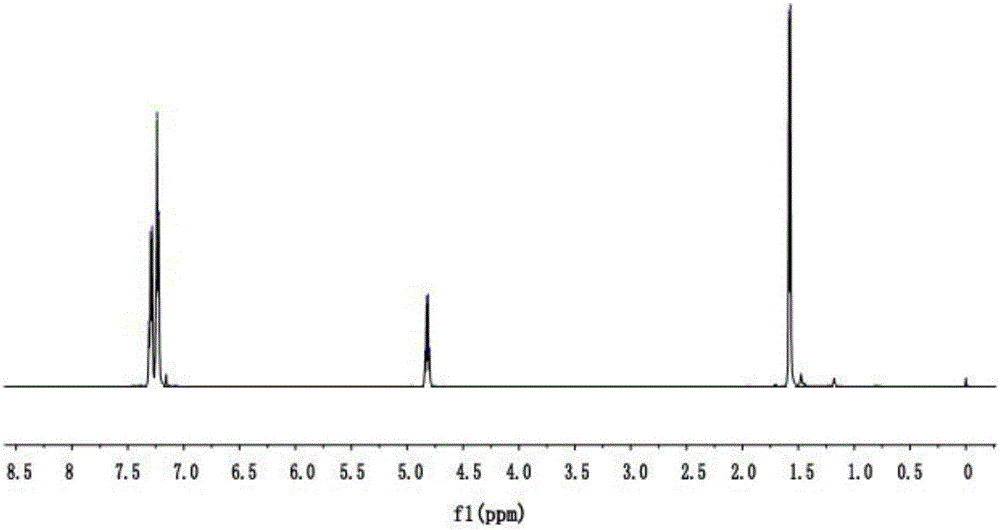
[0036] The nuclear magnetic spectrum data of (S)-1-(1-phenylethyl) isothiocyanate is as image 3 shown.
[0037] 1 H NMR (501MHz, CDCl 3 )δ7.27(dt, J=12.3,7.7Hz,5H),4.82(q,J=6.7Hz,1H),1.58(d,J=6.8Hz,3H).
Embodiment 3
[0039] Preparation of Fe-Anderson Polyoxometalates Modified with One-side Amino Group
[0040] Take 1.5g (1mmol) parent (NH 4 ) 3 (H 2 O) 6 [FeMo 6 o 18 (OH) 6 ]·25H 2 O was dissolved in 10ml deionized water, and after stirring to obtain a light yellow solution, slowly added 0.2g (1.9mmol) trishydroxymethylaminomethane, heated to reflux, and after the reaction was carried out for 24h, 0.9g (3.0mmol) TBAB Add it into the reaction system, and immediately get a large amount of slightly yellow precipitate, that is, the crude product, and filter it with suction to get a light yellow liquid, and place it to get crystals, that is, Fe-Anderson polyacid [TBA] 3 {[NH 2 C(CH 2 O) 3 ]FeMo 6 o 18 (OH) 3}.
[0041] The infrared spectrum of the Fe-Anderson type polyoxometalate modified with one side amino group is as follows: Figure 4 shown.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com