Stable crystalline form I agomelatine tablets and preparation method thereof
A technology for agomelatine tablets and agomelatine, which is applied in the field of pharmaceutical preparations, can solve the problems of inconsistent drug bioavailability, accelerated agomelatine crystal form transformation, and narrow selection of excipients.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
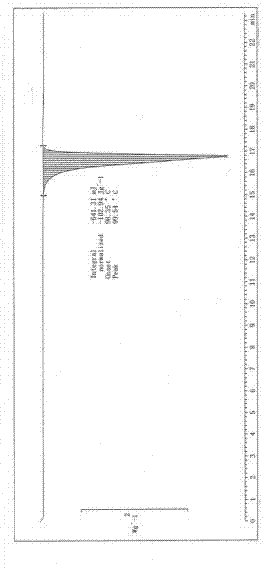
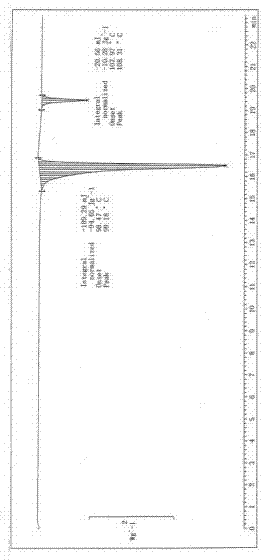
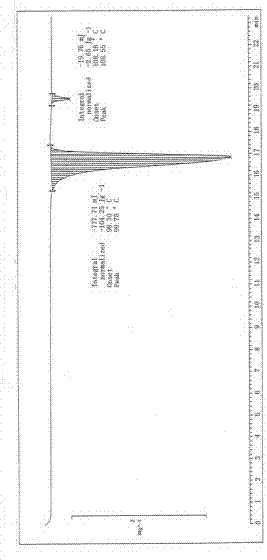
Image
Examples
Embodiment 1
[0135] Agomelatine (Crystal Form I 99%) 25g
[0136] water 20ml
[0137] Lactose 102g
[0138] Hydroxypropyl Cellulose 3g
[0139] Polyvinylpyrrolidone k30 3g
[0140] Croscarmellose Sodium 13g
[0141] Magnesium Stearate 1.3g
[0142] Stearic acid 2.6g
[0143] Silica 0.3g
[0144] Process: Sieve crystal form I agomelatine according to the above weight for later use; take hydroxypropyl cellulose and polyvinylpyrrolidone k30 and stir to dissolve in water (about 40°C), let cool to room temperature, add crystal form I agomelatine Stir the gomelatine well to obtain the protective agent containing crystalline type I agomelatine for later use; then add lactose and part (1 / 2) croscarmellose sodium into the wet mixing granulator and mix evenly , then add the protective agent containing crystalline type I agomelatine, granulate for 2 minutes, and granulate through a swing granulator (833um aperture sieve); fluidized bed drying (inlet air temperature 45°C, boiling bed temperatur...
Embodiment 2
[0146] Agomelatine (more than 90% crystal type I) 25g
[0147] water 30ml
[0148] Lactose 102g
[0149] Hypromellose 4.5g
[0150] Polyvinylpyrrolidone k30 4.5g
[0151] Cross-linked polyvinylpyrrolidone 13g
[0152] Magnesium Stearate 1.3g
[0153] Stearic acid 2.6g
[0154] Silica 0.3g
[0155] Process: Sieve crystal form I agomelatine according to the above weight for later use; take hydroxypropyl methylcellulose and polyvinylpyrrolidone k30 and dissolve them in water (about 40°C), let cool to room temperature, add crystal form I Stir agomelatine evenly; obtain the protective agent containing crystalline type I agomelatine for later use; then add lactose and part (1 / 2) cross-linked polyvinylpyrrolidone into the wet mixing granulator and mix evenly, and then Add the protective agent containing crystalline type I agomelatine, granulate for 2 minutes, and granulate through a swing granulator (833um aperture sieve); fluidized bed drying (inlet air temperature 45°C, boil...
Embodiment 3
[0157] Agomelatine (more than 85% crystal type I) 25g
[0158] water 30ml
[0159] Lactose 99g
[0160] Hypromellose 4.5g
[0161] Hydroxypropyl Cellulose 4.5g
[0162] Croscarmellose Sodium 13g
[0163] Magnesium Stearate 1.3g
[0164] Stearic acid 2.6g
[0165] Silica 0.3g
[0166] Process: Sieve agomelatine crystal I according to the above weight for later use; take hydroxypropyl methylcellulose and hydroxypropyl cellulose and stir to dissolve in water (about 40°C), let cool to room temperature, add crystal I Type agomelatine was stirred evenly; the protective agent containing crystalline type I agomelatine was obtained for later use; then lactose and part (1 / 2) croscarmellose sodium were added to the wet mixing granulator Mix well, then add the protective agent containing crystalline type I agomelatine, granulate for 2 minutes, and granulate through a swing granulator (833um aperture sieve); fluidized bed drying (inlet air temperature 45°C, boiling bed temperature 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com