Methylprednisolone-loading nanoparticles as well as preparation method and application thereof
A technology of nano-microspheres and methylprednisolone, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, and pharmaceutical formulas, which can solve the problem of methylprednisolone’s inability to effectively target delivery, methylprednisolone high Dosage, side effects and other issues, to achieve the effect of prolonging the half-life of the drug, promoting central nervous system damage, and prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
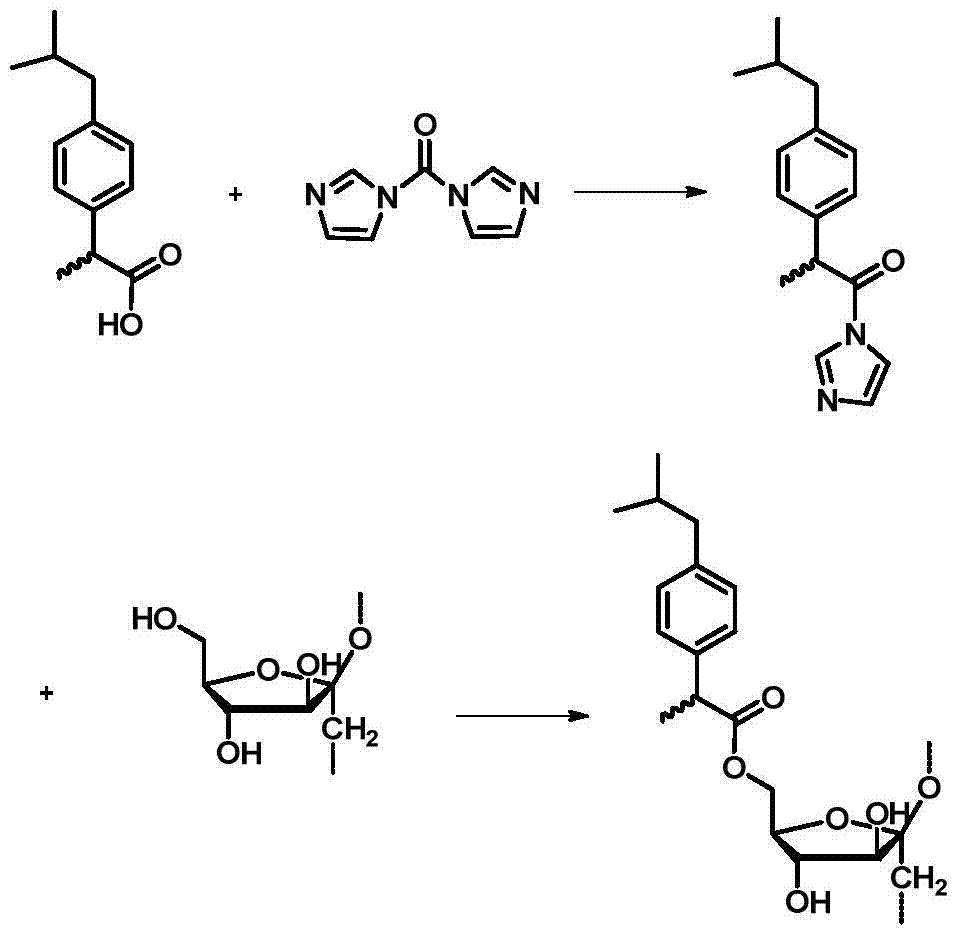
[0020] A method for preparing methylprednisolone-loaded nano-microspheres, using ibuprofen-modified inulin as a carrier, ibuprofen-modified inulin is composed of biocompatible inulin and anti-inflammatory drug ibuprofen, specifically prepared Including the following steps:
[0021] Step 1. Under stirring conditions, add N,N'-carbonyldiimidazole and ibuprofen into dimethyl sulfoxide solvent in a molar ratio of 1-1.3:1, and stir at room temperature for 1-3 hours;
[0022] Step 2, adding inulin to the reaction solution in step 1, and reacting at 60-100° C. for 12-48 hours; wherein, the molar ratio of the inulin unit to the ibuprofen in step 1 is 1-10:2;
[0023] Step 3: Pour the reaction solution in Step 2 into cold water to precipitate precipitates, wash with water, and dry to obtain ibuprofen-modified inulin. The preparation process of ibuprofen modified inulin is as follows:
[0024]
[0025] Step 4. Under stirring conditions, dissolve methylprednisolone and the ibuprofen...
Embodiment 1
[0030] Preparation of Ibuprofen Modified Inulin
[0031] Under stirring conditions, 0.43g N,N'-carbonyldiimidazole (2.65mmol) and 0.5g ibuprofen (2.42mmol) were added to 5mL dimethyl sulfoxide solvent, and the reaction was continued for 1h at room temperature. 0.5g of inulin (3.08mmol) was added to the above reaction solution, and the reaction was stirred at 80°C for 24h. The above reaction solution was poured into 100 mL of cold water, filtered with suction, washed with water, and dried to obtain ibuprofen-modified inulin.
[0032] Preparation of Nanoparticles Loaded with Methylprednisolone
[0033] Under stirring conditions, 5 mg of ibuprofen-modified inulin and 1 mg of methylprednisolone were dissolved in 1 mL of acetone, and added dropwise to 10 mL of 50°C hot water. Stir the above solution overnight to volatilize or evaporate acetone under reduced pressure, freeze-dry to obtain methylprednisolone-loaded nano-microspheres. The particle size range of the nano-microsphere...
Embodiment 2
[0035] Preparation of Ibuprofen Modified Inulin
[0036] Under stirring conditions, 0.43g N,N'-carbonyldiimidazole (2.65mmol) and 0.5g ibuprofen (2.42mmol) were added to 5mL dimethyl sulfoxide solvent, and the reaction was continued for 1h at room temperature. 0.39g of inulin (2.42mmol) was added to the above reaction solution, and stirred and reacted at 80°C for 24h. The above reaction solution was poured into 100 mL of cold water, filtered with suction, washed with water, and dried to obtain ibuprofen-modified inulin.
[0037] Preparation of Nanoparticles Loaded with Methylprednisolone
[0038] Under stirring conditions, 5 mg of ibuprofen-modified inulin and 1 mg of methylprednisolone were dissolved in 1 mL of acetone, and added dropwise to 10 mL of 50°C hot water. The above solution is stirred overnight to volatilize or acetone is aliquoted under reduced pressure, and freeze-dried to obtain methylprednisolone-loaded nano-microspheres. The particle size range of the nano-...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com