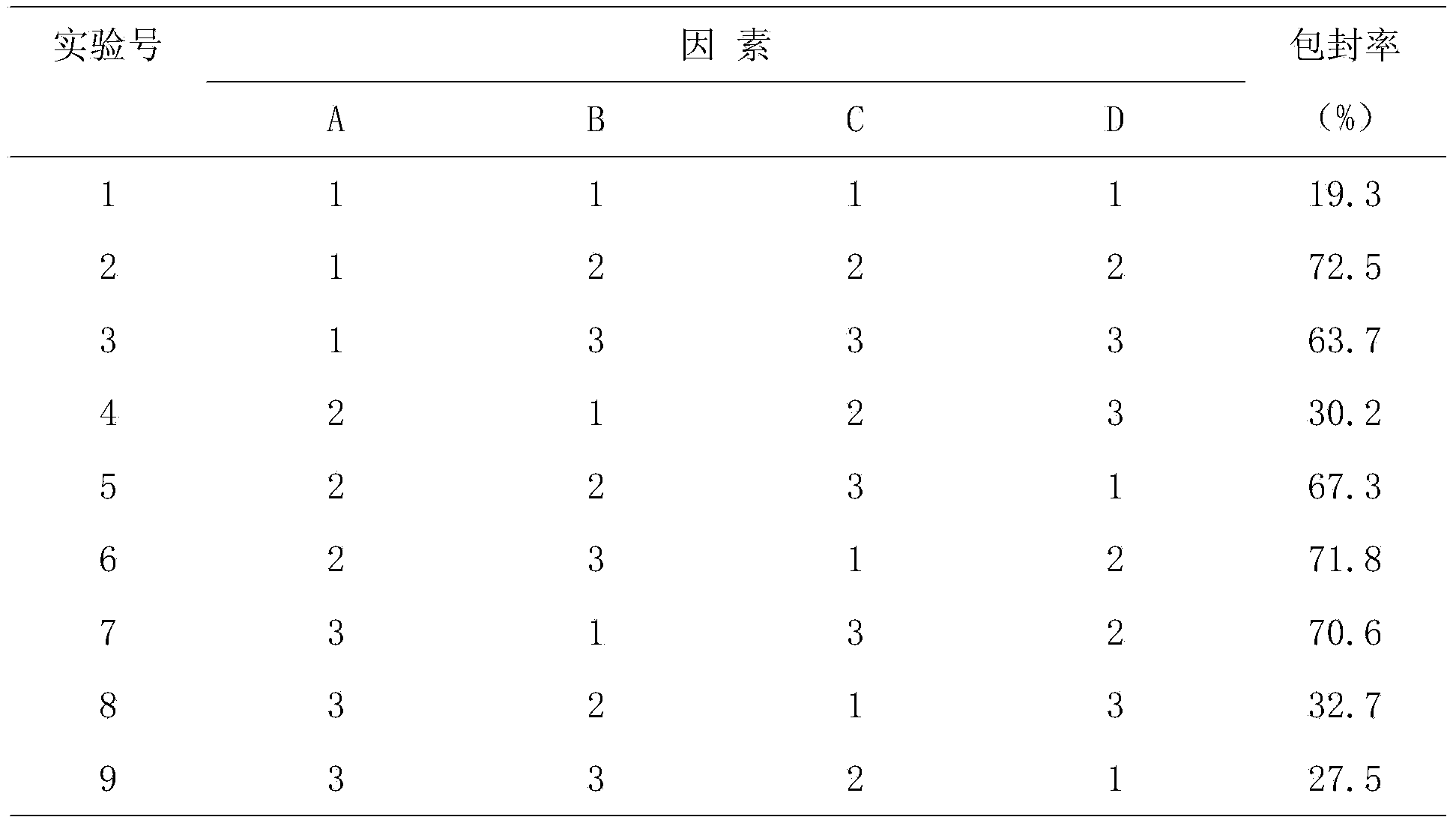Medicine for treating Alzheimer disease and preparation method thereof
A technology for senile dementia and drugs, which is applied in the direction of drug combinations, pharmaceutical formulas, medical preparations containing active ingredients, etc., and can solve problems such as curative effects that need to be further confirmed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
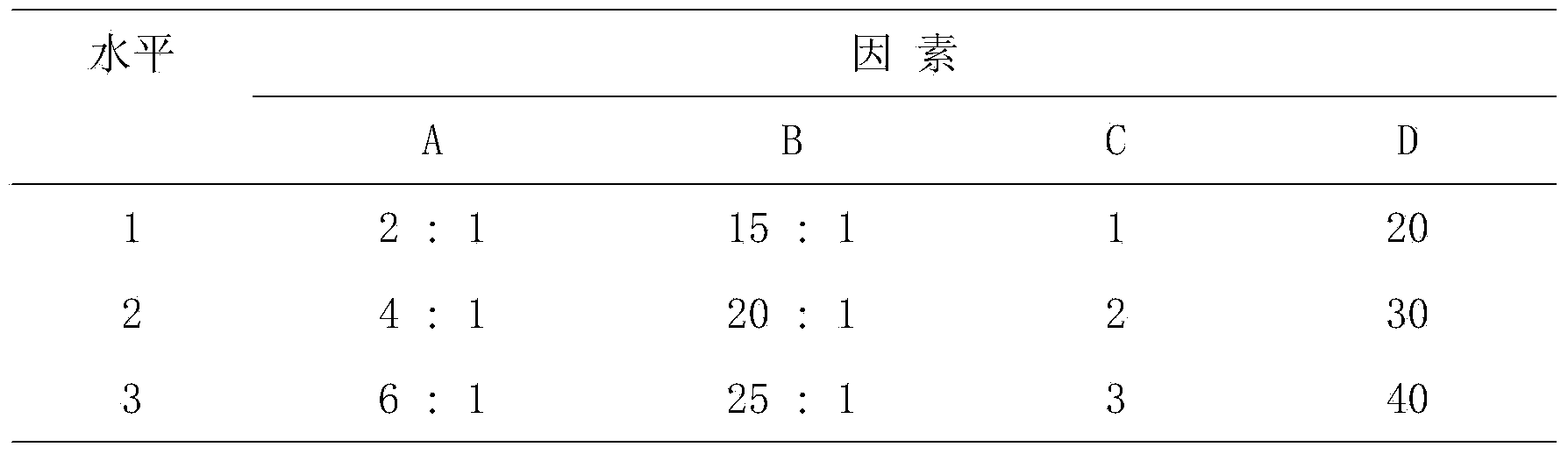
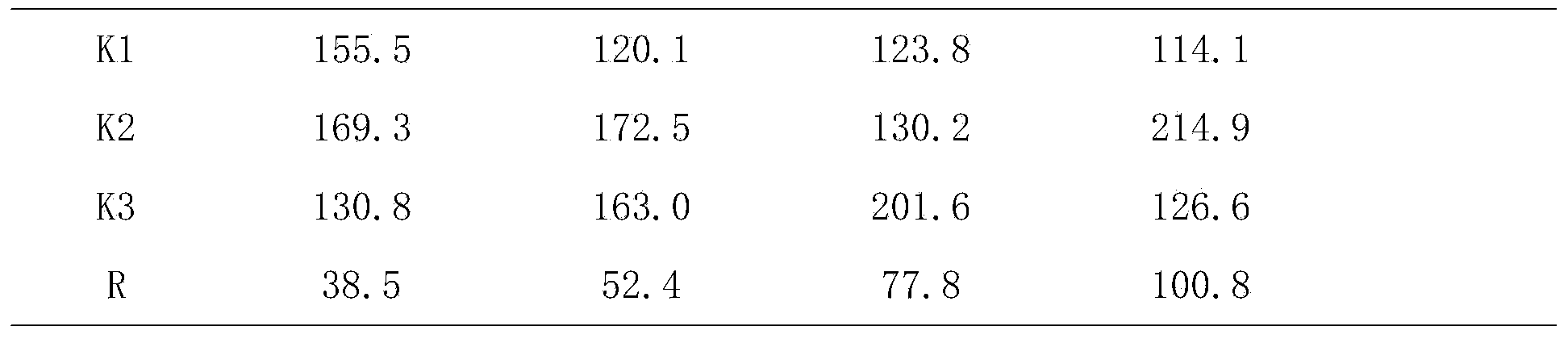
[0084] Formula: 30g of artificial stem cells, 30g of human fetal membranes, 30g of Melaleuca extract, and 10g of DHA.
[0085] Technology: prepare according to the following steps:
[0086] A. Weigh 20 times the amount of lecithin and 5 times the amount of cholesterol in the stem cells, put them in a round bottom flask, add 50 times the amount of absolute ethanol to dissolve the stem cells, and remove the absolute ethanol by rotary evaporation in a water bath at 40°C to make the lipids in the container A layer of uniform film is formed on the wall for later use; take stem cells and put them into a solution containing complete stem cell culture medium, move them to the above-mentioned rotary evaporator with a thin film, hydrate for 30 minutes, and ultrasonicate for 15 minutes with an ice-water bath probe to obtain stem cell lipids. Plastid suspension, the suspension was frozen at -20°C for 24 hours, then thawed at room temperature and then frozen at -20°C for 24 hours, repeated...
Embodiment 2
[0092] Formula: 50g of artificial stem cells, 50g of human fetal membranes, 50g of Melaleuca extract, and 20g of DHA.
[0093] Technology: prepare according to the following steps:
[0094] A. Weigh 20 times the amount of lecithin and 5 times the amount of cholesterol in the stem cells, put them in a round bottom flask, add 50 times the amount of absolute ethanol to dissolve the stem cells, and remove the absolute ethanol by rotary evaporation in a water bath at 40°C to make the lipids in the container A layer of uniform film is formed on the wall for later use; take stem cells and put them into a solution containing complete stem cell culture medium, move them to the above-mentioned rotary evaporator with a thin film, hydrate for 30 minutes, and ultrasonicate for 15 minutes with an ice-water bath probe to obtain stem cell lipids. Plastid suspension, the suspension was frozen at -20°C for 24 hours, then thawed at room temperature and then frozen at -20°C for 24 hours, repeated...
Embodiment 3
[0100] Formula: 10g of artificial stem cells, 10g of human fetal membranes, 10g of Melaleuca extract, 5g of DHA.
[0101] Technology: prepare according to the following steps:
[0102] A. Weigh 20 times the amount of lecithin and 5 times the amount of cholesterol in the stem cells, put them in a round bottom flask, add 50 times the amount of absolute ethanol to dissolve the stem cells, and remove the absolute ethanol by rotary evaporation in a water bath at 40°C to make the lipids in the container A layer of uniform film is formed on the wall for later use; take stem cells and put them into a solution containing complete stem cell culture medium, move them to the above-mentioned rotary evaporator with a thin film, hydrate for 30 minutes, and ultrasonicate for 15 minutes with an ice-water bath probe to obtain stem cell lipids. Plastid suspension, the suspension was frozen at -20°C for 24 hours, then thawed at room temperature and then frozen at -20°C for 24 hours, repeated free...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com