Process for producing pharmaceutical auxiliary material hydroxypropyl methylcellulose through slurry process
A technology of hypromellose and pharmaceutical excipients, which is applied in the field of hypromellose slurry production process of pharmaceutical excipients, can solve the problems of less specifications, yellowish color, and large fluctuations in product viscosity, and achieve The effect of increasing output, large single batch output, and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
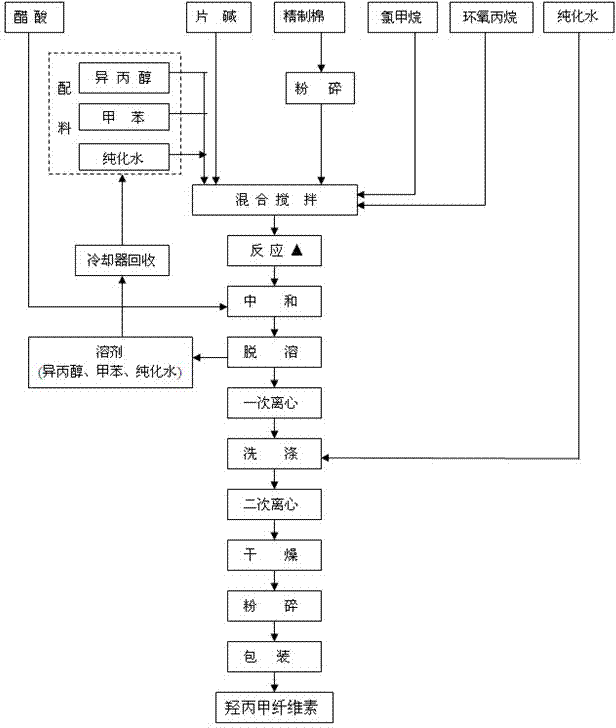
[0022] The production process of hypromellose slurry method for pharmaceutical excipients comprises the following steps:
[0023] (1) Ingredients: Mix toluene, isopropanol, and purified water evenly. The parts by weight of the toluene are 82 parts, the parts by weight of isopropanol are 18 parts, and the parts by weight of purified water are 2.6 parts. Distribution is made into a transparent and non-turbid mixed solvent; the parts described below are parts by weight;
[0024] Add 7000 parts of the above mixed solvent to the reaction kettle, add purified water, the total amount of purified water is 360-440 parts, put 800 parts of caustic soda into the reaction kettle, then raise the temperature to 80°C, keep it for 30min, and then immediately cool down To 26°C, add 800 parts of refined cotton to the reaction kettle; the bulk density of refined cotton is ≥160g / L, and the transmittance of 80 mesh is ≥90%;
[0025] (2) Start timing from the time of adding refined cotton, stir for...
Embodiment 2
[0032] The production process of hypromellose slurry method for pharmaceutical excipients comprises the following steps:
[0033] (1) Ingredients: Mix toluene, isopropanol, and purified water evenly. The parts by weight of the toluene are 80 parts, the parts by weight of isopropanol are 15 parts, and the parts by weight of purified water are 2.5 parts. Distribution is made into a transparent and non-turbid mixed solvent; the parts described below are parts by weight;
[0034] Add 7000 parts of the above-mentioned mixed solvent to the reaction kettle, add purified water, the total amount of purified water is 360 parts, put 780 parts of caustic soda into the reaction kettle, then raise the temperature to 75°C, keep it for 25min, and then immediately cool down to 25°C ℃, add 780 parts of refined cotton to the reactor;
[0035] (2) Reaction: Start timing from the time of adding refined cotton, stir for 15 minutes, heat up to 80°C, time for 8 minutes, then cool down to 22°C, time ...
Embodiment 3
[0041] The production process of hypromellose slurry method for pharmaceutical excipients comprises the following steps:
[0042] (1) Ingredients: Mix toluene, isopropanol, and purified water evenly. The parts by weight of the toluene are 85 parts, the parts by weight of isopropanol are 20 parts, and the parts by weight of purified water are 3.5 parts. Distribution is made into a transparent and non-turbid mixed solvent; the parts described below are parts by weight;
[0043]Add 7000 parts of the above-mentioned mixed solvent to the reaction kettle, add purified water, the total amount of purified water is 440 parts, put 820 parts of caustic soda into the reaction kettle, then raise the temperature to 85°C, keep it for 35min, and then immediately cool down to 30°C ℃, add 820 parts of refined cotton to the reactor;
[0044] (2) Reaction: Start timing from the time of adding refined cotton, stir for 30 minutes, heat up to 90°C, time for 12 minutes, then cool down to 26°C, time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com