Methods for preparing pleuripotent cardiovascular progenitor cells and maintaining cardiovascular differentiation capacity
A technology of precursor cells and pluripotent stem cells, which can be applied to non-embryonic pluripotent stem cells, artificially induced pluripotent cells, animal cells, etc., and can solve the problems of little understanding of regulation and molecular basis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
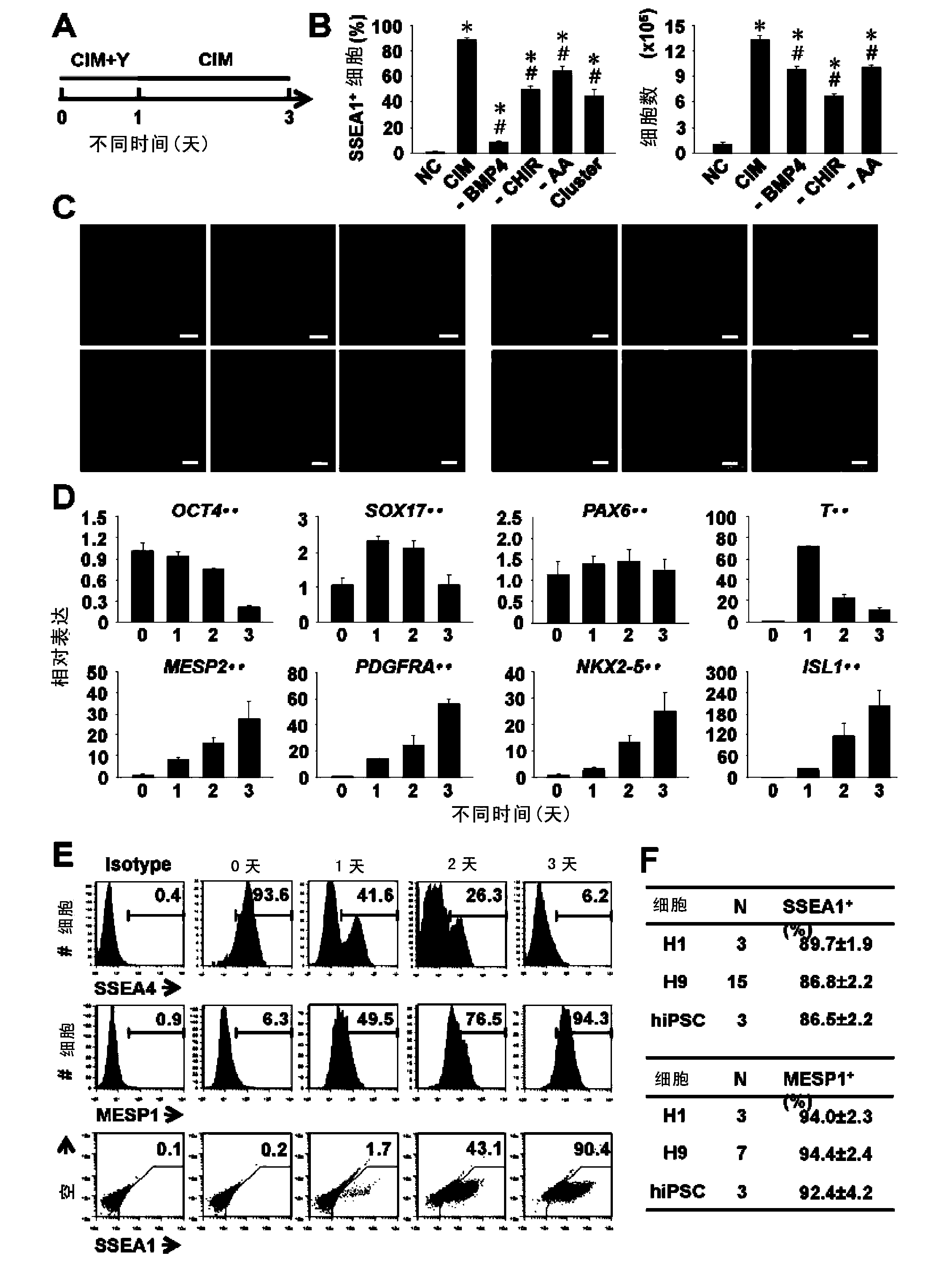
[0085] The present invention also includes the cell group containing cardiovascular precursor cells obtained by the aforementioned preparation method. In the cell group, the number of cardiovascular precursor cells accounts for more than 70% of the total number of cells, preferably more than 80% of the total number of cells; more preferably more than 85% of the total number of cells (by surface markers SSEA1 or MESP1 positive cells count).
[0086] Once a cell population containing cardiovascular precursor cells has been obtained, the identification of cardiovascular precursor cells can be achieved using RT-PCR and other gene expression analysis techniques.
[0087] Cardiovascular precursor cells can be identified or isolated or enriched using methods known to those skilled in the art. More classical methods such as fluorescence-activated cell sorting (FACS) or magnetic cell sorting, that is, the use of antibodies against specific surface molecules of cardiovascular precursor...
Embodiment 1
[0193] Example 1. Efficient transformation of human pluripotent stem cells (hPSCs) into homogeneous CVPCs
[0194] To induce hPSCs towards a cardiovascular fate, the inventors screened important signal transduction pathways during mesoderm formation and subsequent cardiac differentiation, including Wnt, GSK3, FGF, BMP and Activin / Nodal. In order to make the conditions uniform, the inventors used a single cell-based differentiation method on the basis of a monolayer culture system, and added the Rho kinase inhibitor Y27632 in the first day of differentiation to improve the survival of the cells. After a series of systematic attempts, the inventors found that the joint addition of 25ng / ml BMP4, 50 μg / ml AA and 3 μM GSK3 inhibitor CHIR99021 (CHIR) (the mixture of these combined reagents is named CIM) can efficiently remove untreated drugs within 3 days. The differentiated human embryonic stem cell line H9 converted into a homogeneous cell population that mostly (88.9 ± 1.8%) expr...
Embodiment 2
[0197] Example 2. Long-term maintenance of self-renewal of hPSC-derived CVPCs
[0198] Proliferation and lineage determination of CVPCs are regulated by a delicate microenvironment and complex levels of multiple signal transduction pathways, including Wnt, FGF, BMP, Notch, Hedgehog, vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF ), MEK, retinoic acid (RA), and Activin / Nodal. Therefore, under specific conditions in vitro, it is generally difficult to maintain the self-renewal and expansion of CVPCs. The inventors hypothesized that if the conditions that induce CVPC differentiation are concertedly eliminated, they should be able to maintain basal state self-renewal and continued proliferation. To verify it, the inventors selected 9 signaling pathway inhibitors together with the GSK3 inhibitor CHIR (CHIR99021) (Table 3) to verify their role in stimulating CVPC expansion. Clonogenicity was not observed in H9-derived CVPCs on day 3 of differentiat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com