A kind of preparation method of gefitinib form 1 crystal form
A technology of gefitinib and crystal form, which is applied in the field of crystal form preparation of pharmaceutical compounds, can solve problems such as changes in dissolution rate and bioavailability of active compounds, unfavorable product quality, etc., and achieve the goal of avoiding dissolution rate and bioavailability change, benefit quality control, and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
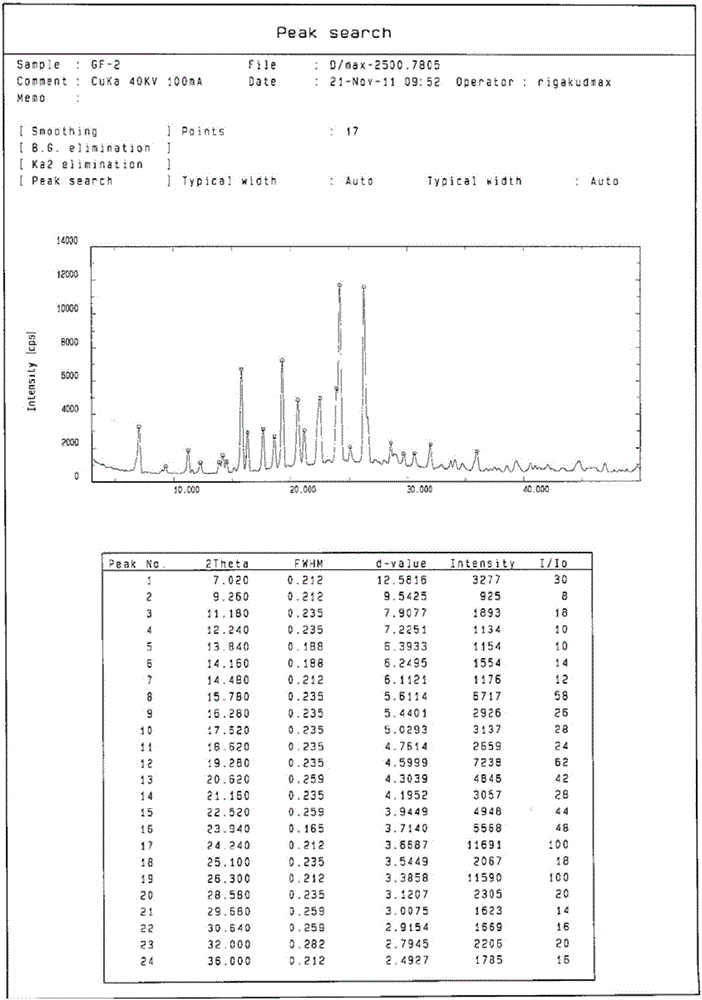
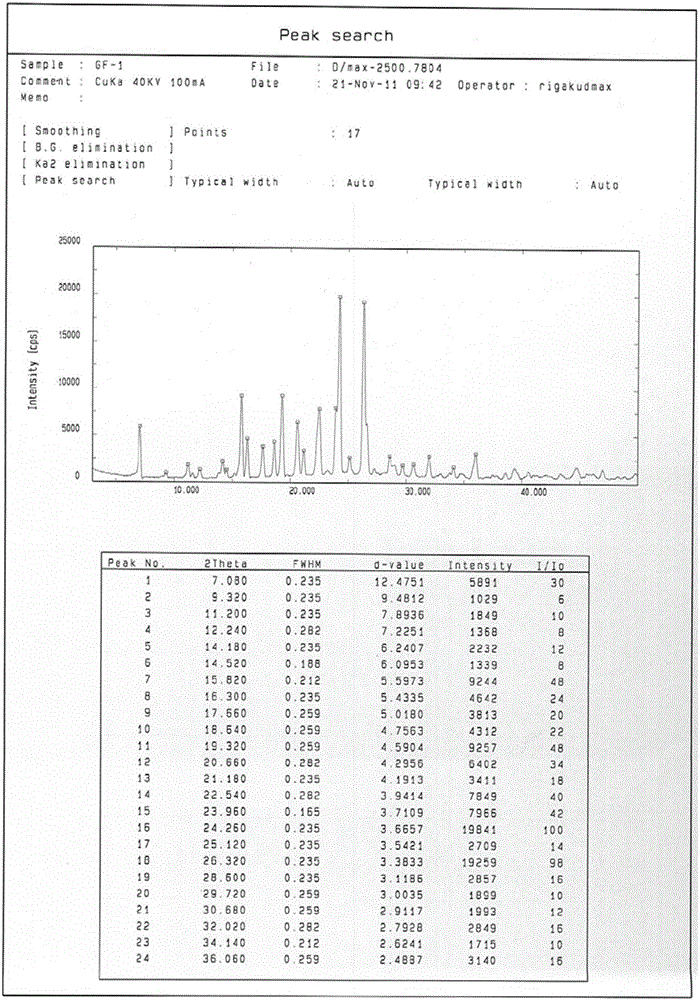
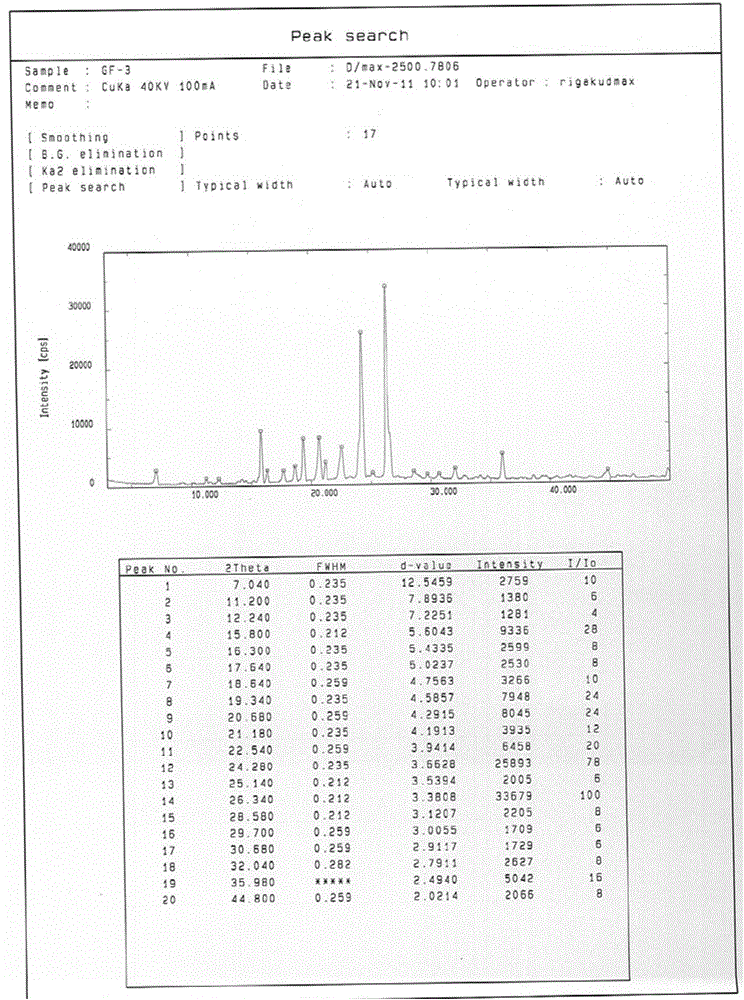
[0052]Add 1g of gefitinib into 20ml of acetone, control the temperature at 60°C, stir to dissolve all, and slowly cool down to room temperature after dissolving, collect the solid by filtration, wash with cold acetone (3×5mL), and dry in vacuum. According to X-ray diffraction analysis, it is Form1 crystal form of gefitinib. A total of 900 mg of Form1 crystal form of gefitinib was obtained, with a yield of 90%.
Embodiment 2
[0054] Add 1g of gefitinib into 20ml of 2-butanone, control the temperature at 70°C, stir to dissolve all, slowly cool down to room temperature after dissolving, collect the solid by filtration, wash with cold 2-butanone (3×5mL), vacuum After drying, the obtained crystals were analyzed by X-ray diffraction to be Form 1 crystal form of gefitinib. A total of 920 mg of Form 1 crystal form of gefitinib was obtained, with a yield of 92%.
Embodiment 3
[0056] Add 1g of gefitinib into 10ml of 2-pentanone, control the temperature at 90°C, stir to dissolve it all, slowly cool down to room temperature after dissolving, collect the solid by filtration, wash with cold 2-pentanone (3×5mL), vacuum After drying, the obtained crystals were analyzed by X-ray diffraction to be Form 1 crystal form of gefitinib. A total of 960 mg of Form1 crystal form of gefitinib was obtained, with a yield of 96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com