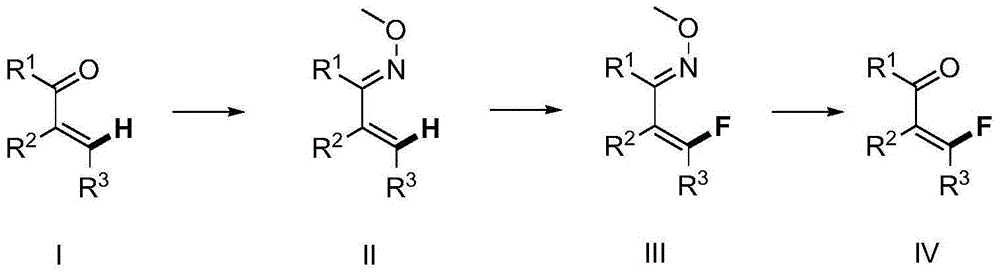
A method for synthesizing β-fluoro-α, β-unsaturated enone compounds
An unsaturated compound technology, applied in the hydrolysis of carbonyl compounds, organic chemistry, oxime preparation, etc., to achieve high fluorination selectivity, mild reaction conditions, and good substrate adaptability.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043]
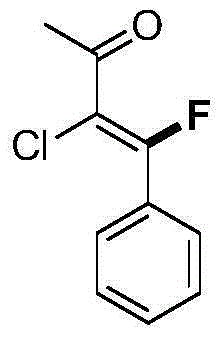
[0044] [1] Mix 0.900g (5.0mmol) of 3-chloro-4-phenylbutenone, 0.835g (10.0mmol) of methoxyamino hydrochloride, 1.640g (20.0mmol) of anhydrous sodium acetate, 10ml of ethanol and 30ml of water was added to the 100ml flask. After the mixture was heated to reflux for 2 hours, TLC detected that the reaction was complete, and 30 ml of ethyl acetate was added for dilution and extraction. The organic layer was dried and the solvent was removed under reduced pressure to obtain 0.867 g of 3-chloro-4-phenylbutenone oxime ether ( 83% yield).
[0045] [2] Add 3-chloro-4-phenylbutenone oxime ether (62.7mg, 0.3mmol) and tris(dibenzylideneacetone)dipalladium (13.7mg, 0.015mmol) into a closed reaction vessel, N-fluorobenzenesulfonimide (189.0mg, 0.6mmol), potassium nitrate (9.1mg, 0.09mmol), nitromethane (3.0mL), the reaction mixture was stirred at 25°C, followed by TLC detection, and the reaction was complete within 24 hours . Stop the reaction, dilute the mixture with dichlor...
Embodiment 2
[0048]
[0049] [1] Mix 0.900g (5.0mmol) of 3-chloro-4-phenylbutenone, 0.835g (10.0mmol) of methoxyamino hydrochloride, 1.640g (20.0mmol) of anhydrous sodium acetate, 10ml of ethanol and 30ml of water was added to the 100ml flask. After the mixture was heated to reflux for 2 hours, TLC detected that the reaction was complete, and 30ml of ethyl acetate was added for dilution and extraction. The organic phase was dried and the solvent was removed under reduced pressure to obtain 0.867g of 3-chloro-4-phenylbutenone oxime ether ( 83% yield).
[0050] [2] Add 3-chloro-4-phenylbutenone oxime ether (62.7mg, 0.3mmol) and tris(dibenzylideneacetone)dipalladium (13.7mg, 0.015mmol) into a closed reaction vessel, N-fluorobenzenesulfonylimide (189.0mg, 0.6mmol), silver nitrate (15.2mg, 0.09mmol), nitromethane (3.0mL), the reaction mixture was stirred at 25°C, followed by TLC detection, and the reaction was complete within 24 hours . Stop the reaction, dilute the mixture with dichlorom...
Embodiment 3
[0053]
[0054] [1] Mix 0.900g (5.0mmol) of 3-chloro-4-phenylbutenone, 0.835g (10.0mmol) of methoxyamino hydrochloride, 1.640g (20.0mmol) of anhydrous sodium acetate, 10ml of ethanol and 30ml of water was added to the 100ml flask. After the mixture was heated to reflux for 2 hours, TLC detected that the reaction was complete, and 30 ml of ethyl acetate was added for dilution and extraction. The organic layer was dried and the solvent was removed under reduced pressure to obtain 0.867 g of 3-chloro-4-phenylbutenone oxime ether ( 83% yield).
[0055] [2] Add 3-chloro-4-phenylbutenone oxime ether (62.7mg, 0.3mmol), palladium diacetate (6.7mg, 0.03mmol), N-fluorobenzenesulfonyl Imine (189.0mg, 0.6mmol), potassium nitrate (9.1mg, 0.09mmol), nitromethane (3.0mL), the reaction mixture was stirred at 25°C, followed by TLC detection, and the reaction was complete within 24 hours. Stop the reaction, dilute the mixture with dichloromethane, filter and remove the solvent under reduce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com