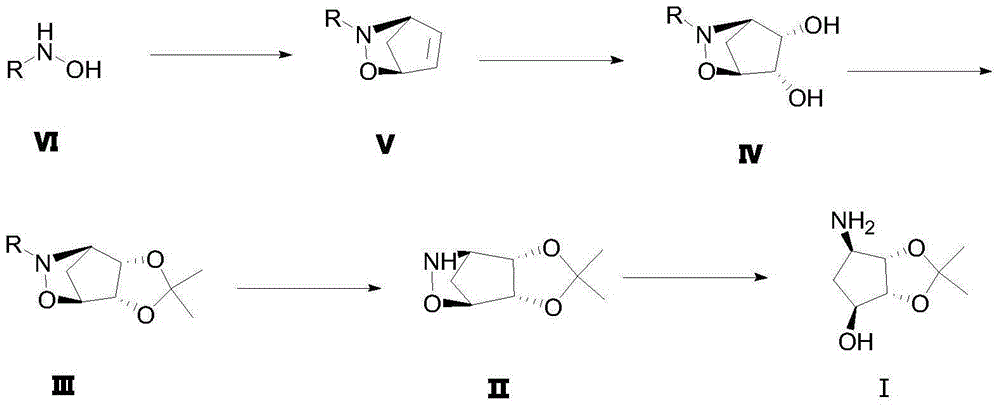
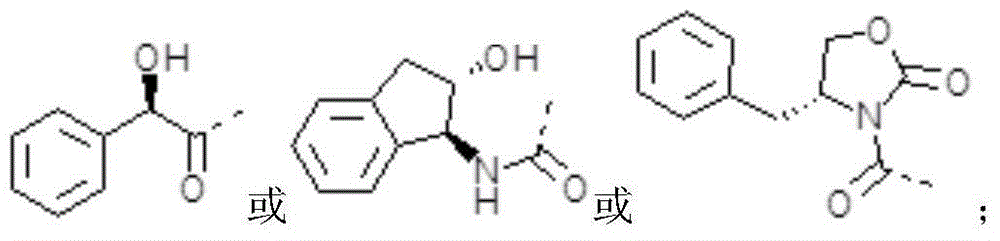
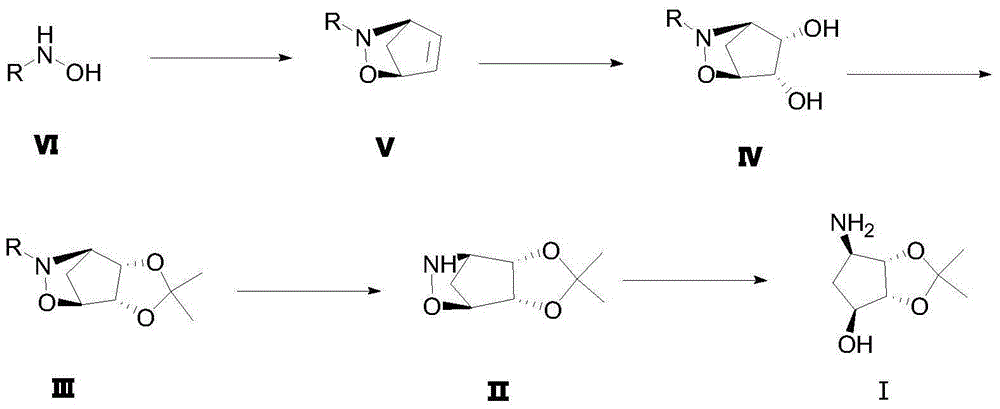
A method for preparing (1s, 2r, 3s, 4r)-2,3-o-isopropylidene-4-aminocyclopentane-1,2,3-triol
A technology of aminocyclopentane and isopropylidene, which is applied in the chemical industry, can solve the problems of non-recycling and increased production costs, and achieve the effects of increased yield, low price, and reduced costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] (1) Synthesis of R-ethyl mandelate
[0049]
[0050] Weigh 10.5g R-mandelic acid into a reaction bottle filled with 50ml of absolute ethanol, then slowly add 2ml of concentrated sulfuric acid along the wall, reflux at 80°C for 4 hours, remove the solvent by rotary evaporation, then add an appropriate amount of dichloromethane, and wash with water Twice, the organic layer was dried with anhydrous sodium sulfate, filtered and spin-dried to obtain 12 g of product, yield: 96.46%.
[0051] (2) Synthesis of compound VI
[0052]
[0053] Weigh 12g of potassium hydroxide and dissolve it in 40ml of anhydrous methanol at room temperature, pour 9g of hydroxylamine hydrochloride into a solution of 60ml of anhydrous methanol under nitrogen protection, a large amount of white solids are produced, filter, and the filtrate is cooled to 0 degrees; Measure 12g of R-methyl mandelate into the reaction flask, stir overnight at room temperature, remove the solvent by rotary evaporatio...
Embodiment 2
[0062]
[0063] For the specific preparation method, refer to Example 1.
Embodiment 3
[0065]
[0066] For the specific preparation method, refer to Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com