Oligopeptide with breast cancer resisting activity and application thereof
An anti-breast cancer, breast cancer cell technology, applied to oligopeptide with anti-breast cancer activity and its application, oligopeptide and its application field, can solve the problems of high cost and long cycle, achieve small molecular weight and easy industrialization , the effect of inhibiting tumor proliferation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Design and synthesis of oligopeptides
[0025] 1. Extract the major international antimicrobial peptide database (http: / / aps.unmc.edu / AP / main.php; http: / / penbase.immunaqua.com / ; http: / / www.bbcm.univ.trieste.it / ; Http: / / www.imtech.res.in / raghava / antibp / ), analyze the corresponding relationship between its primary structure and activity, and design the artificial short peptide sequence.
[0026] 2. Use the protein analysis database http: / / www.expasy.org / to analyze the similarity of the secondary structure between the oligopeptide sequence with known activity and the designed oligopeptide sequence, and to modify the design sequence in combination with other physical and chemical parameter predictions .
[0027] 3. Send the oligopeptide sequence to the biological company for synthesis or use an automatic oligopeptide synthesizer to synthesize the full sequence. The purity of HPLC detection is greater than 96%, and the quality of oligopeptide synthesis is determined by mass spe...
Embodiment 2
[0031] Identification of oligopeptides
[0032] P1222 oligopeptide was hydrolyzed by 5.7mol / L HCl for 16 hours, and analyzed for amino acid. It was found that it contained 9 kinds of amino acids including Phe, Thr, Gln, Tyr, Pro, Lys, Arg, Gly, Ser, etc. The molar ratio was Phe: Thr : Gln: Tyr: Pro: Lys: Arg: Gly: Ser = 1:1:1:1:1:1:1:1:1. After amino acid sequence analysis, the sequence is Phe-Thr-Gln-Tyr-Pro-Lys-Arg-Gly-Ser. A peak was determined by electrospray mass spectrometry, and its molecular weight was 1083.21.
Embodiment 3
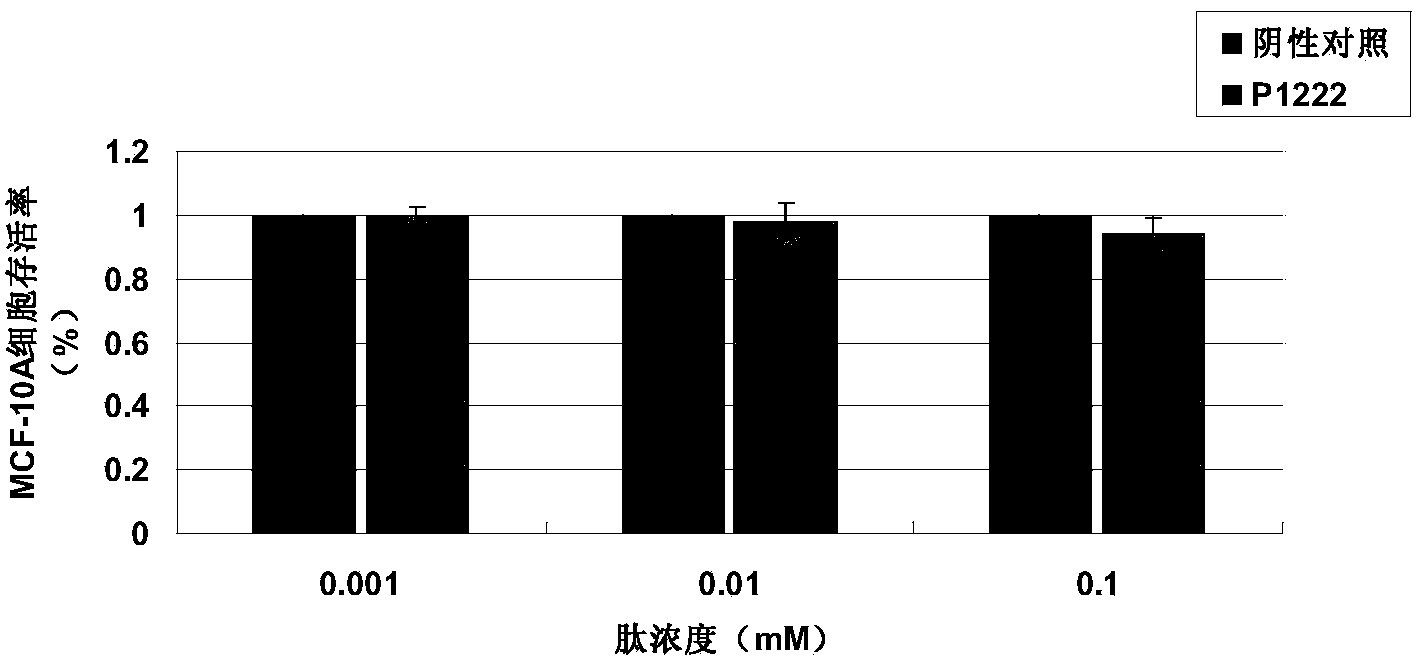
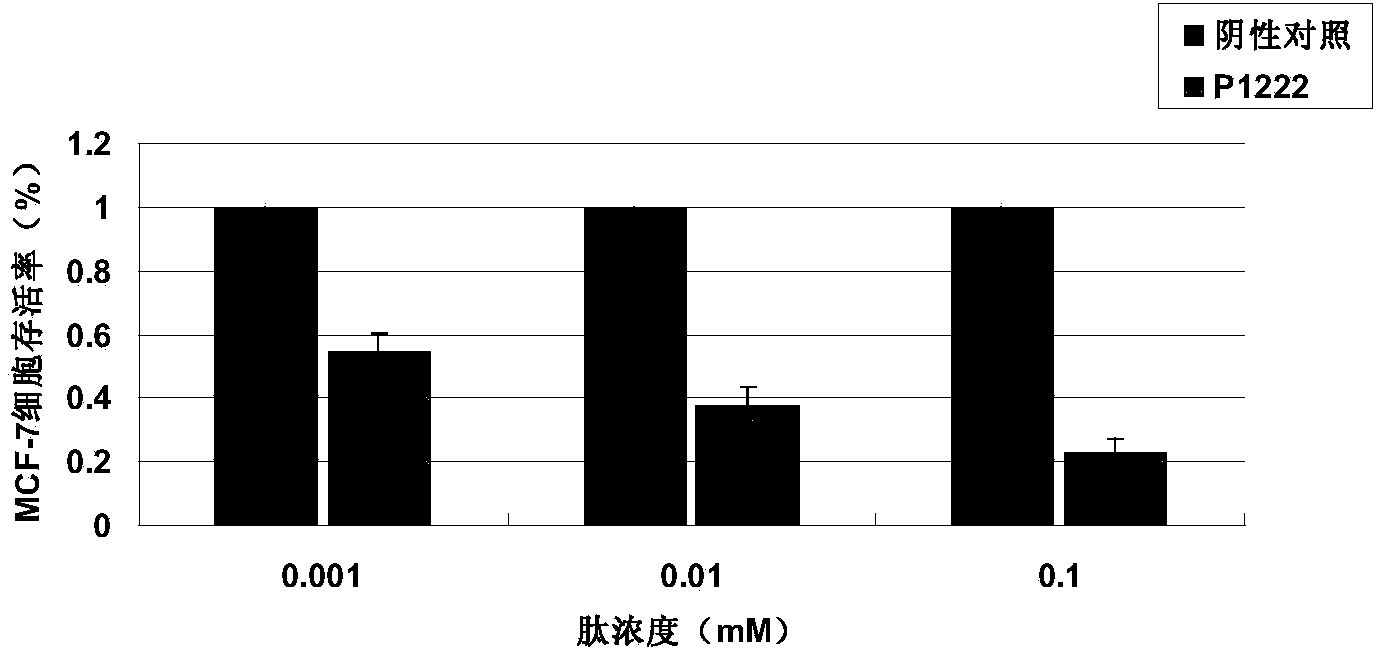
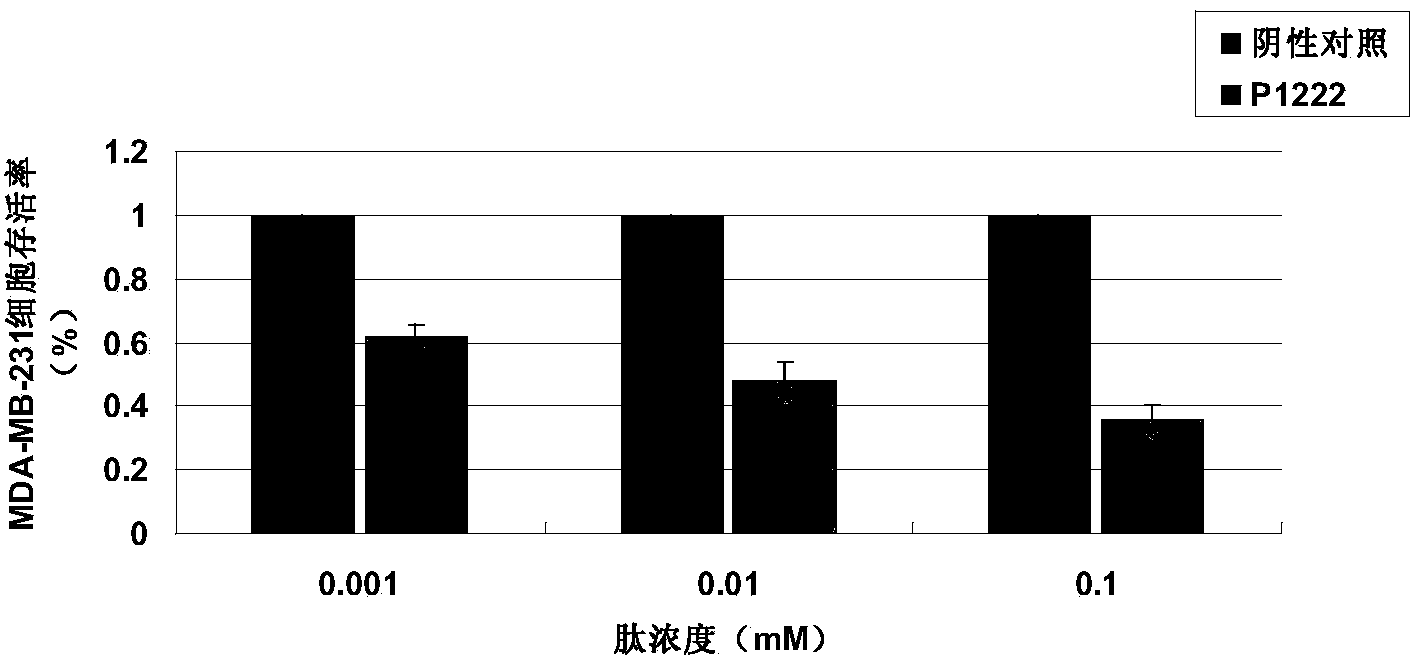
[0034] Detection of tumor suppressor activity of P1222 oligopeptide in vitro by MTT method
[0035] 2.1 Materials and reagents
[0036] 1) P1222 oligopeptide, synthesized by Shanghai Shenggong Biological Engineering Technology Service Co., Ltd.
[0037] 2) Human breast cancer cell lines MCF-7 and MDA-MB-231, human normal breast cells MCF-10A, were purchased from the Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences.
[0038] 3) RPMI1640 medium, DMEM medium, and newborn calf serum are all products of GIBCO, USA.
[0039] 4) Dimethyl sulfoxide (DMSO), tetramethyl azoazole (MTT) and trypsin are products of Sigma.
[0040] 2.2 Experiment
[0041] 1) Dilute P1222 oligopeptide to an initial concentration of 1 mM with a diluent (sterile PBS, or sterile water, or corresponding cell culture medium), filter and sterilize, and freeze at -20°C after aliquoting.
[0042] 2) Cell culture: The cancer cells are cultured in RPMI1640 culture medium (containing ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com