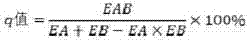Silibinin and sorafenib-containing pharmaceutical composition and applications thereof
A technology of silibinin and composition, which is applied in the field of pharmaceutical compositions containing silybin and sorafenib, can solve the problems of combined drug use and no silybin, and achieve good synergy and bioavailability The effect of improving the degree of toxicity and small side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1 The determination and IC of silybin and Sorafenib combined drug concentration 50 Determination.
[0024] In this example, the MTT method was used to analyze the drug sensitivity of HepG2 cells to silybin and sorafenib. HepG2 cells were treated with 1×10 4 / mL concentration was inoculated in a 96-well culture plate, 100 μL / well, and after culturing overnight, different concentrations of drugs or control solutions (20 μL per well) were added. Among them, silybin had 8 concentration gradients, respectively 10, 5 , 2.5, 1.25, 0.625, 0.3125, 0.1500 and 0.0750 (units are μmol / L); Sorafenib has 7 concentration gradients, respectively 10, 5, 2.5, 1.25, 0.625, 0.3125 and 0.1500 (units are μmol / L). Three replicate wells were set for each drug concentration, and the experiment was repeated three times, and a drug-free control group was also set. After culturing for 24h, 48h, and 72h, add 20μL of MTT solution to each well, and continue to culture for 4h. Add 130μL ...
Embodiment 2
[0027] Example 2 Experiment of the inhibitory rate of the pharmaceutical composition on the proliferation of HepG2 cells.
[0028] HeG2 cells in logarithmic phase were divided into 1×10 4 The density per well was inoculated in a 96-well plate, 50 μL / well, and drugs were added after 24 hours of culture (50 μL of silibinin, sorafenib, combined group or control solution was added to each well). Among them, the concentrations of silybin were 0.01, 0.05, 0.1, 0.2, 0.4, 0.8 and 1.0 (units are μmol / L), and the concentrations of sorafenib were 0.01, 0.1, 0.2, 0.4, 0.8, 1.6 and 2.0 (units are μmol / L), the concentration of the combination group is (0.01 μmol / L silybin + 0.01 μmol / L sorafenib), (0.05 μmol / L silybin + 0.1 μmol / L sorafenib) fenib), (0.1 μmol / L silybin + 0.2 μmol / L sorafenib), (0.2 μmol / L silybin + 0.4 μmol / L sorafenib), (0.4 μmol / L water Silybin + 0.8μmol / L sorafenib), (0.8μmol / L silybin + 1.6μmol / L sorafenib) and (1.0μmol / L silybin + 2.0μmol / L sora Fini), and each conc...
Embodiment 3
[0034] Example 3 In vitro inhibition experiments of individual drugs and combined drugs on various tumor cells.
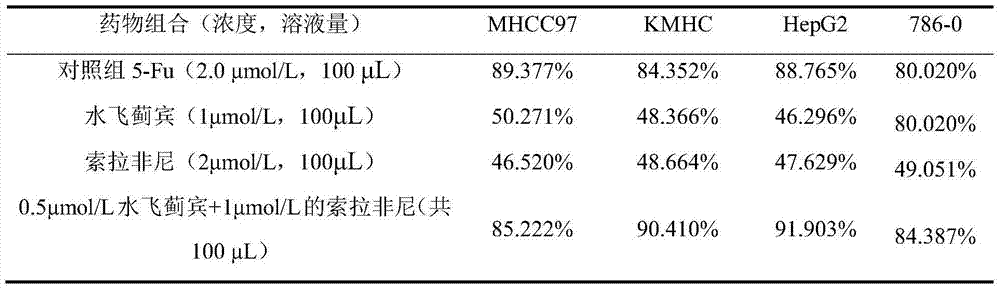
[0035] The MTT method was used to determine the concentration of silybin (1 μmol / L), sorafenib (2 μmol / L) alone and the combination of both (0.5 μmol / L silybin + 1 μmol / L sorafenib) within 72 hours. Inhibitory effect on the proliferation of various liver cancer cells (MHCC97, KMHC and HepG2 cells) and renal cancer cells (786-0 cells). The test results are shown in Table 2. Compared with the single drug, the combined drug (0.5 μmol / L silybin + 1 μmol / L sorafenib) has better effects on various malignant tumor cells, especially for those with HepG2 cells, which are closer to primary liver cancer cells, have a better inhibitory effect.
[0036] Table 2 Sorafenib, silybin and the inhibitory effect of the combination of the two on cancer proliferation (measurement time 72 hours)
[0037]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com