Use of a liquid allergy vaccine formulation for oromucosal administration
A liquid vaccine, oral mucosal technology, applied in the directions of allergen antigen components, medical preparations with non-active ingredients, drug combinations, etc. Simple effect of drug regimen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] Example 1: Sublingual immunotherapy (SLIT) without dose escalation ( ) - new treatment options
[0085] introduction
[0086] Over the past few years, several studies have been conducted using sublingual immunotherapy (SLIT) generating evidence of efficacy combined with a favorable safety profile (1).
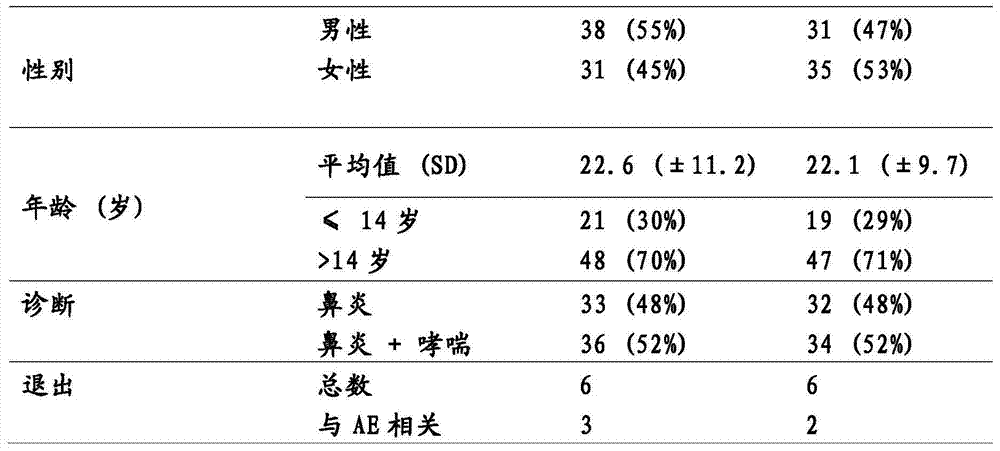
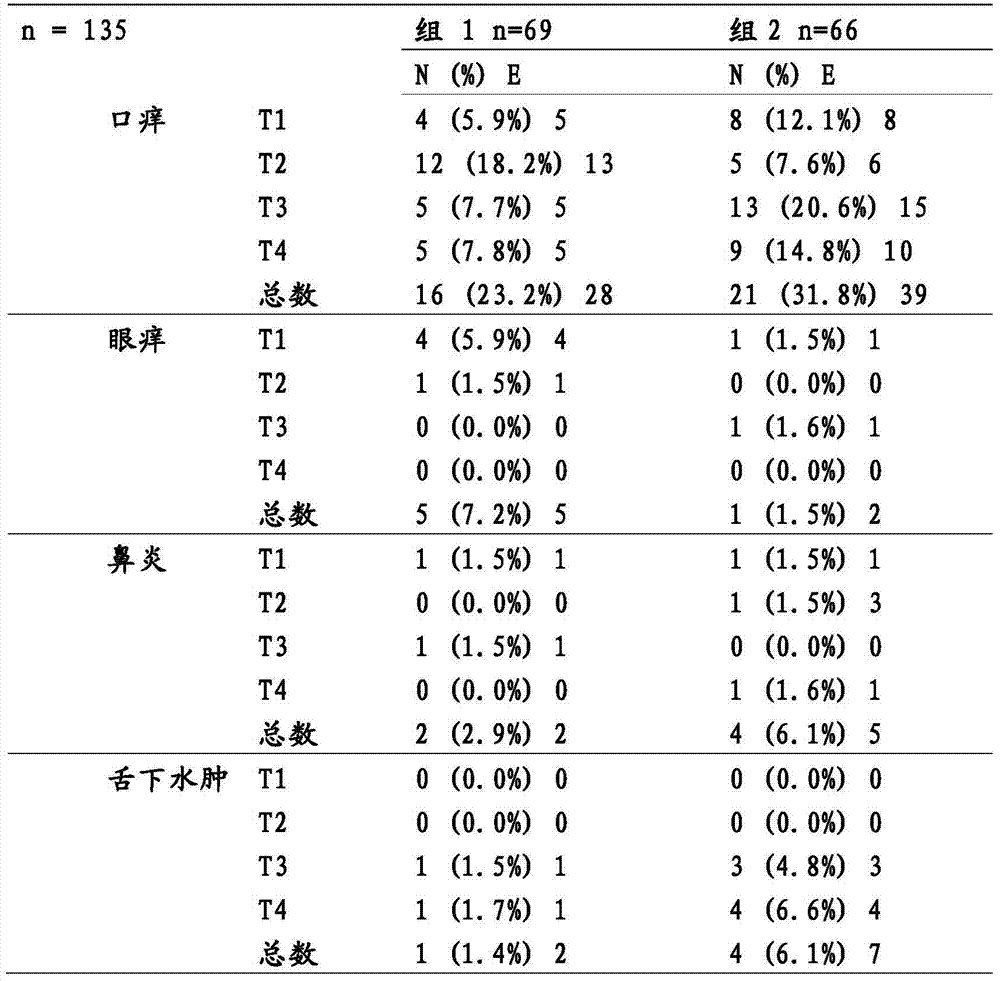
[0087] In order for patients to obtain high levels of self-administered therapies such as effect, the compliance must be high (2). Given that the basis for compliance is convenience, it is relevant to enhance this property of the product. The excellent tolerability of SLIT has led to consideration of eliminating the possibility of increasing dose periods. This would improve patient convenience, which could increase compliance and potentially increase treatment efficiency. This study is designed to evaluate Tolerability in patients with mite or grass sensitization without dose escalation.
[0088] Materials and methods
[0089] potion
[0090] (ALK-Abell...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com