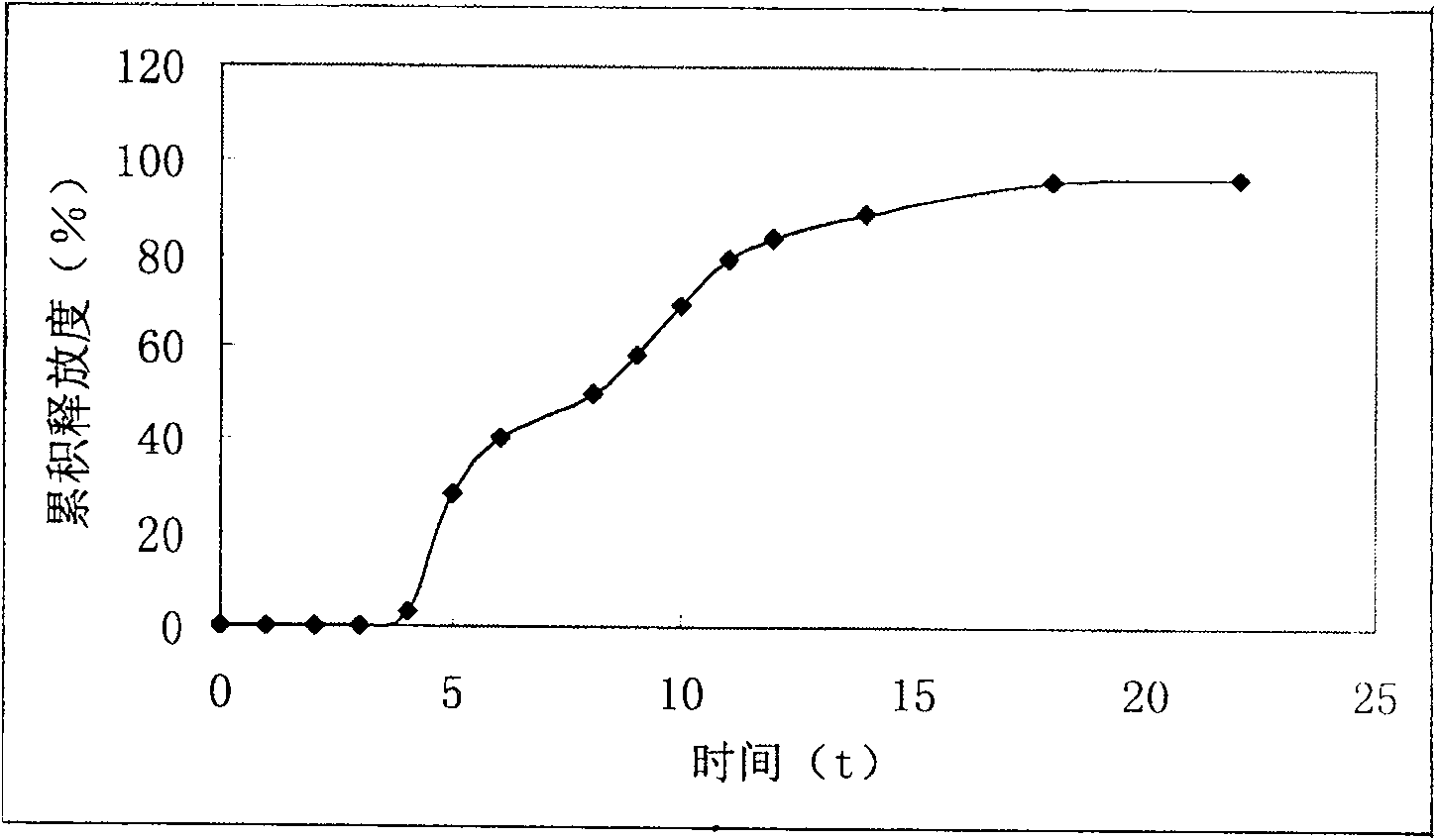
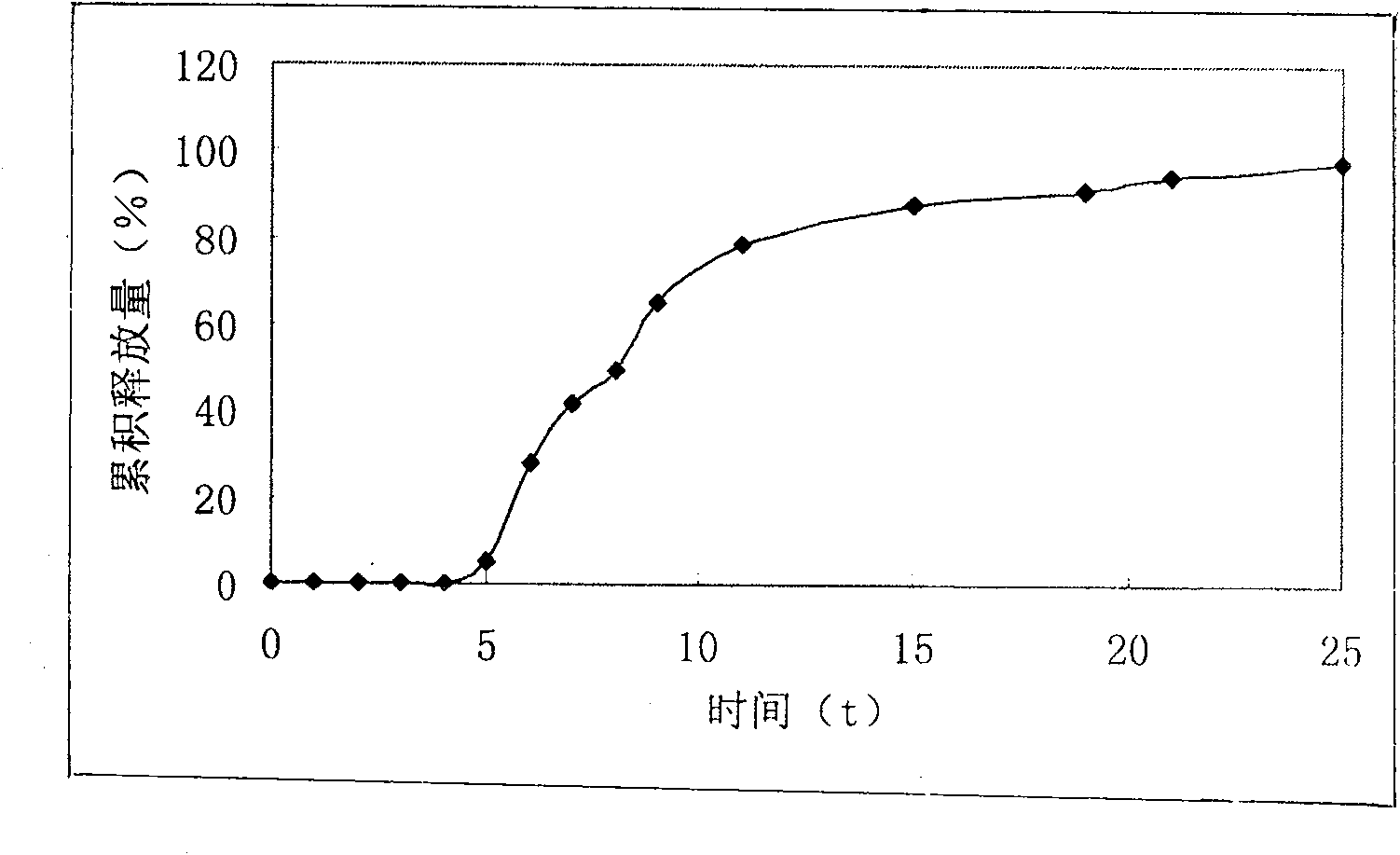
Isosorbide mononitrate timing quick-release and slow-release preparation
A slow-release and preparation technology, applied in the field of isosorbide mononitrate time-release sustained-release preparations, to achieve the effects of improving stability, avoiding drug resistance, and preventing drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Isosorbide Mononitrate 7.7g
[0052] Microcrystalline shot core (25 / 30m) 200g
[0053] HPMC E5 0.77g
[0055] EC 9.6g
[0056] 80% ethanol 120g
[0057] Eudragit RS30D 235.1g (acrylic resin accounts for 70.53g)
[0058] Eudragit RL30D 57.6g (acrylic resin accounted for 17.28g)
[0059] Triethyl citrate 8.8g
[0060] Glyceryl monostearate 4.3g
[0061] Isosorbide Mononitrate 3.4g
[0062] Low-substituted hydroxypropyl cellulose 52.5g
[0063] HPMC E5 26.2g
[0064] Surelease (water dispersion of ethyl cellulose) 1070g (ethyl cellulose accounts for 267.5g)
[0066] Preparation:
[0067] Add 0.77g of HPMC (E5) into 200g of warm water at 90°C under stirring. After swelling, slowly add 7.7g of isosorbide mononitrate, stir, and the solution becomes clear, then add 0.2g of talc, stir until completely dispersed, and set aside. Weigh 9.6 g of EC and add it to 120 g of 80% ethanol, stir for 45 minutes to obtain a c...
Embodiment 2
[0070] Isosorbide Mononitrate 158g
[0071] Microcrystalline shot core (25 / 30m) 200g
[0072] PVP K30 19.8g
[0074] HPMC E5 9.6g
[0075] Eudragit NE30D 186.6g (acrylic resin accounted for 55.98g)
[0076] Eudragit RS30D 8.3g (acrylic resin accounted for 2.49g)
[0077] Polyethylene glycol 0.83g
[0078] Talc powder 33.2g
[0079] Isosorbide Mononitrate 67g
[0080] Crospovidone xL-10 57.7g
[0081] PVP K30 144.3g
[0082] Glyceryl monostearate 342.9g
[0083] Talc powder 1.41g
[0084] Preparation:
[0085] Add 19.8g of PVP K30 into 200g of warm water under stirring, after swelling, slowly add 158g of isosorbide mononitrate, stir, the solution becomes clear, then add 0.2g of talc, stir until completely dispersed, set aside. Weigh 9.6g of HPMC E5 and add it into 120g of water, and wait for swelling to prepare a coating solution for subsequent use. Eudragit NE30D 186.6g, Eudragit RL30D 8.3g are fully stirred, add polyethylene glycol 0.83g...
Embodiment 3
[0088] Isosorbide Mononitrate 35g
[0089] Sucrose core (40 / 50m) 200g
[0090] HPMC E5 87.5g
[0091] Talc powder 0.2g
[0092] Hydroxypropyl Cellulose 56g
[0093] Methylcellulose 22.4g
[0094] EC 344g
[0095] 80% ethanol 425g
[0096] Diethyl phthalate 68g
[0097] Isosorbide Mononitrate 15g
[0098] Croscarmellose Sodium 373g
[0099] Methylcellulose 37.3g
[0100] Eudragit RS30D 345.6g (acrylic resin accounted for 103.68g)
[0101] Eudragit RL30D 84.7g (acrylic resin accounted for 25.41g)
[0102] Triethyl citrate 12.7g
[0103] Glyceryl monostearate 6.36g
[0104] Tween 80 3.5g
[0105] Preparation:
[0106] Add 87.5g of HPMC (E5) into 200g of warm water at 90°C under stirring. After swelling, slowly add 35g of isosorbide mononitrate, stir, and the solution becomes clear, then add 0.2g of talc, stir until completely dispersed, and set aside. Weigh 56 g of hydroxypropyl cellulose and add it into 120 g of 95% alcohol water, and stir for 45 minutes to obtain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com