A kind of ophthalmic composition for improving the stability of chloramphenicol and preparation method thereof
A technology of ophthalmic composition and chloramphenicol, which is applied in the direction of non-active ingredient medical preparations, medical preparations containing active ingredients, drug combinations, etc., can solve the problem of improving the stability of preparations and improve treatment compliance stability, improving stability, and reducing the number of administrations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0027] The embodiment of the present invention also proposes a preparation method of the ophthalmic composition for improving the stability of chloramphenicol, comprising the following steps:
[0028] Inject the prepared water for injection into the batching tank, add preservatives, pH regulators, osmotic pressure regulators and polyoxyethylene sorbitan monooleate into the batching tank according to the proportion, stir until completely dissolved, and boil; Dissolve chloramphenicol in castor oil, stir until dissolved, add into the batching tank; stir evenly, add the remaining amount of water for injection, sterilize and filter, fill, and the part amount of water for injection is 65% to 75% of the total amount.
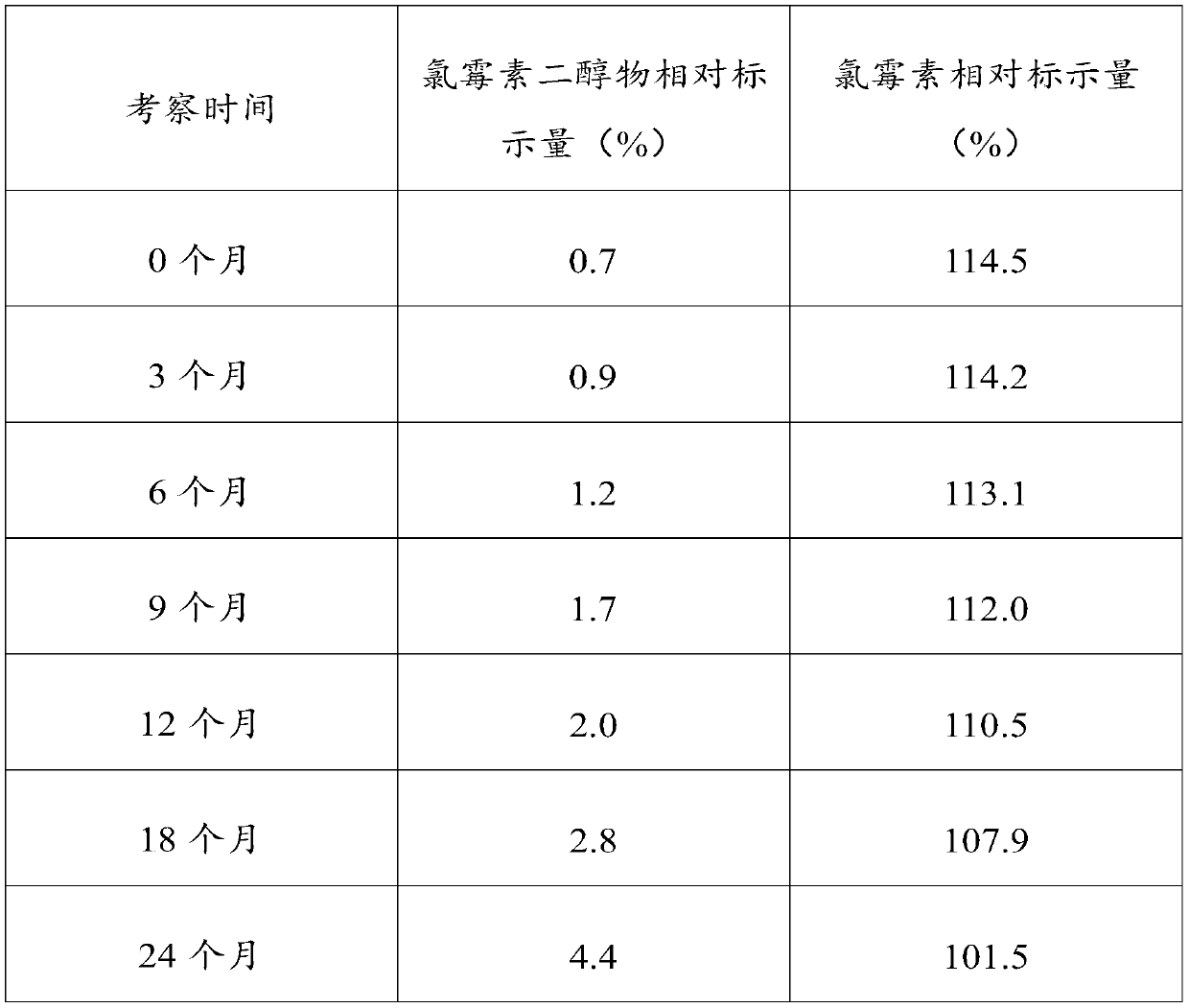
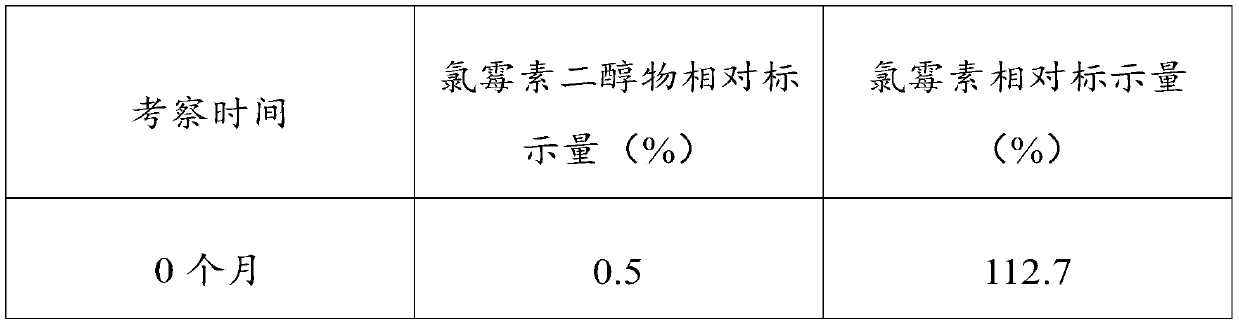
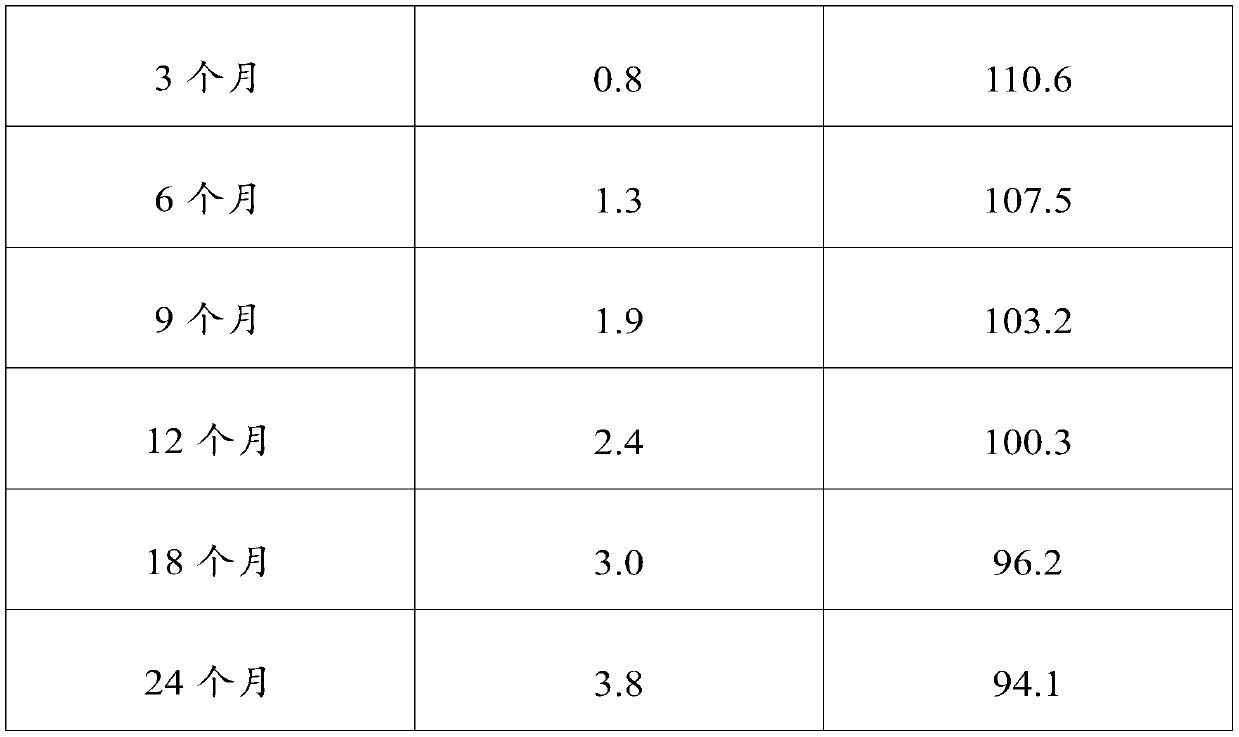
[0029] It is generally believed that when the pH of chloramphenicol eye drops is below 7, the amide is hydrolyzed to generate amino and dichloroacetic acid. In the range of pH 2-7, pH has little effect on the rate of hydrolysis. It is most stable at pH 6, the hydrolys...
Embodiment 1
[0034] An ophthalmic composition for improving the stability of chloramphenicol, comprising the following components: 0.1% chloramphenicol, 0.7% pH regulator, 0.01% preservative, 0.3% osmotic pressure regulator, 1.2% castor oil and Polyoxyethylene sorbitan monooleate 0.5%, the balance is water for injection.
Embodiment 2
[0036]An ophthalmic composition for improving the stability of chloramphenicol, comprising the following components: 0.2% chloramphenicol, 1.0% pH regulator, 0.02% preservative, 0.4% osmotic pressure regulator, 1.8% castor oil and Polyoxyethylene sorbitan monooleate 0.6%, the balance is water for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com