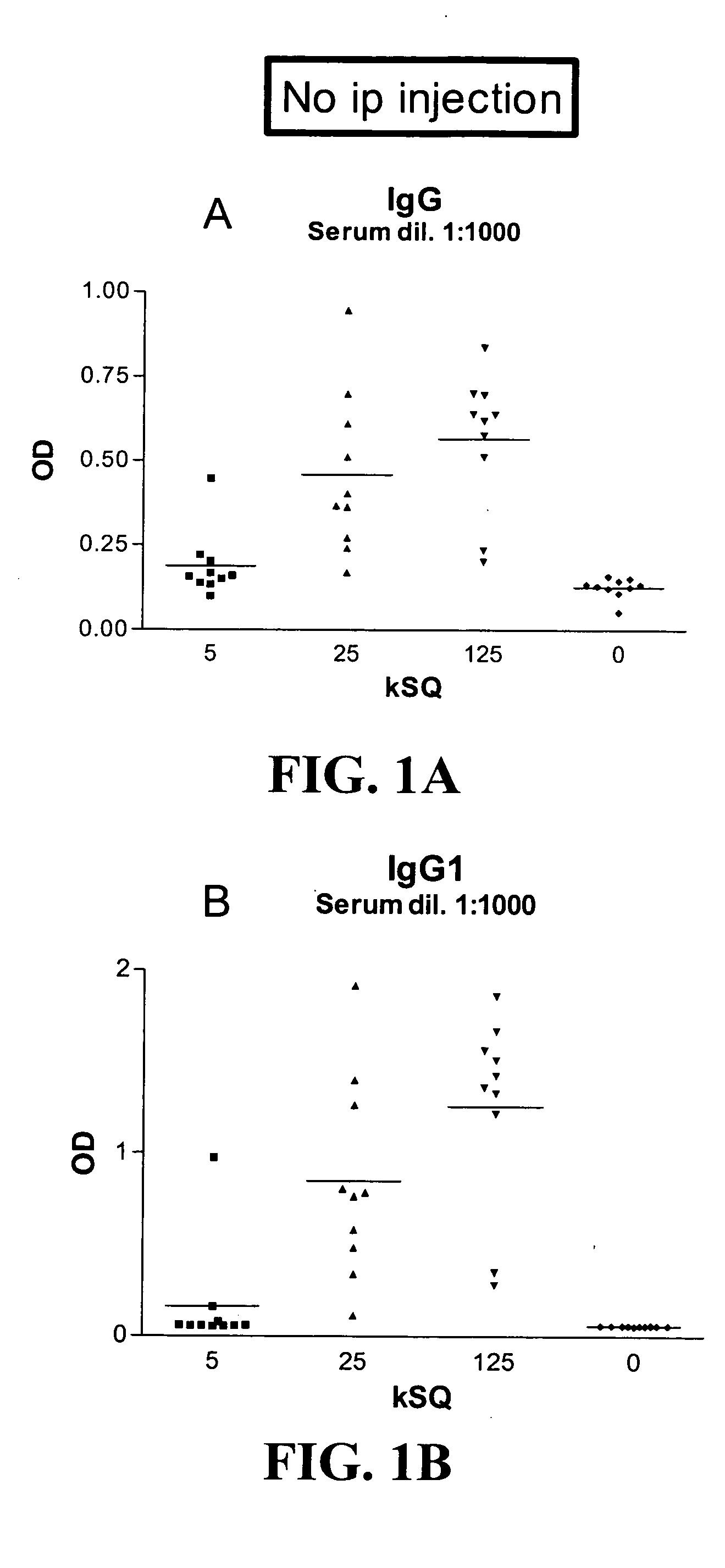
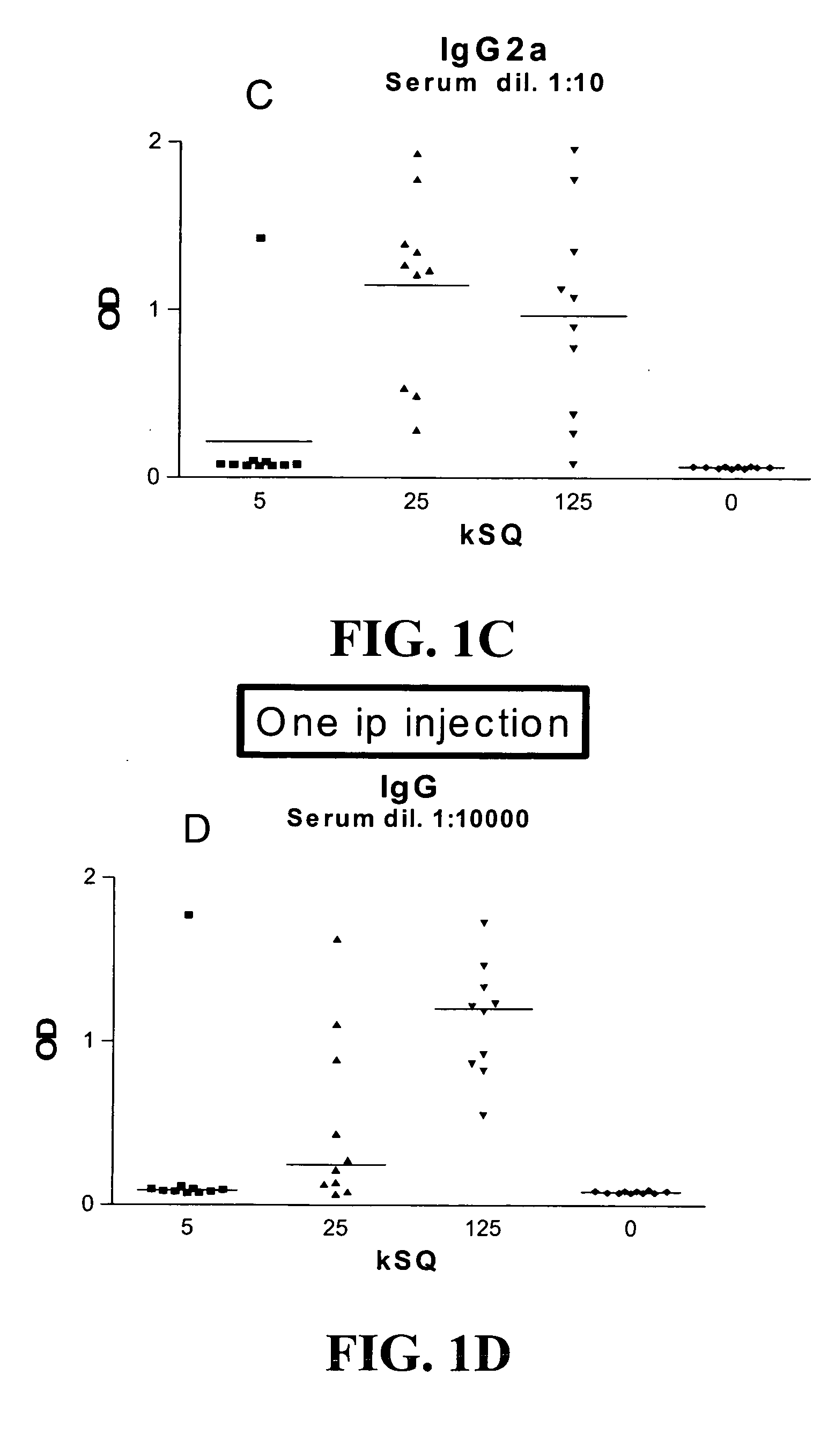
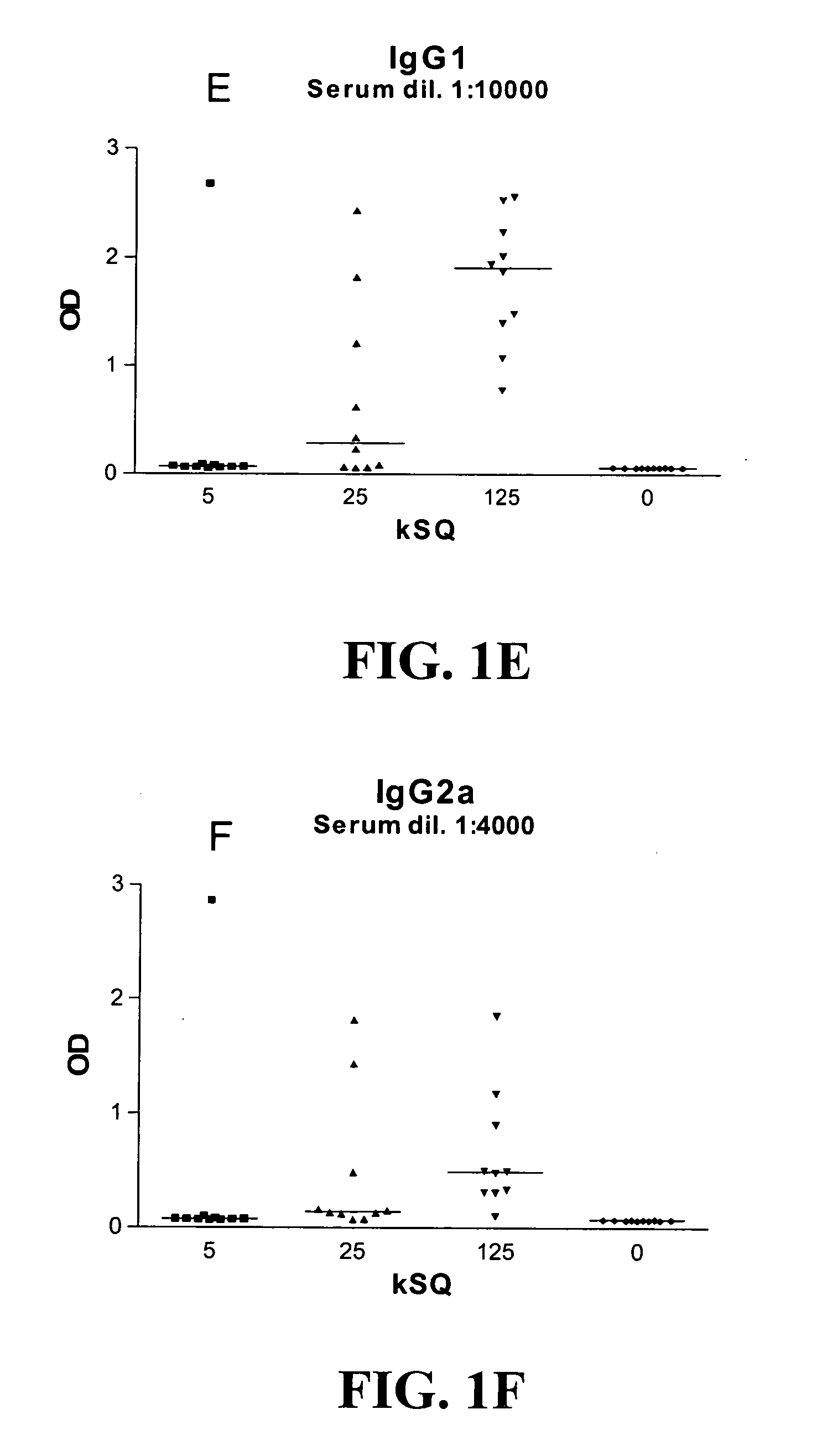
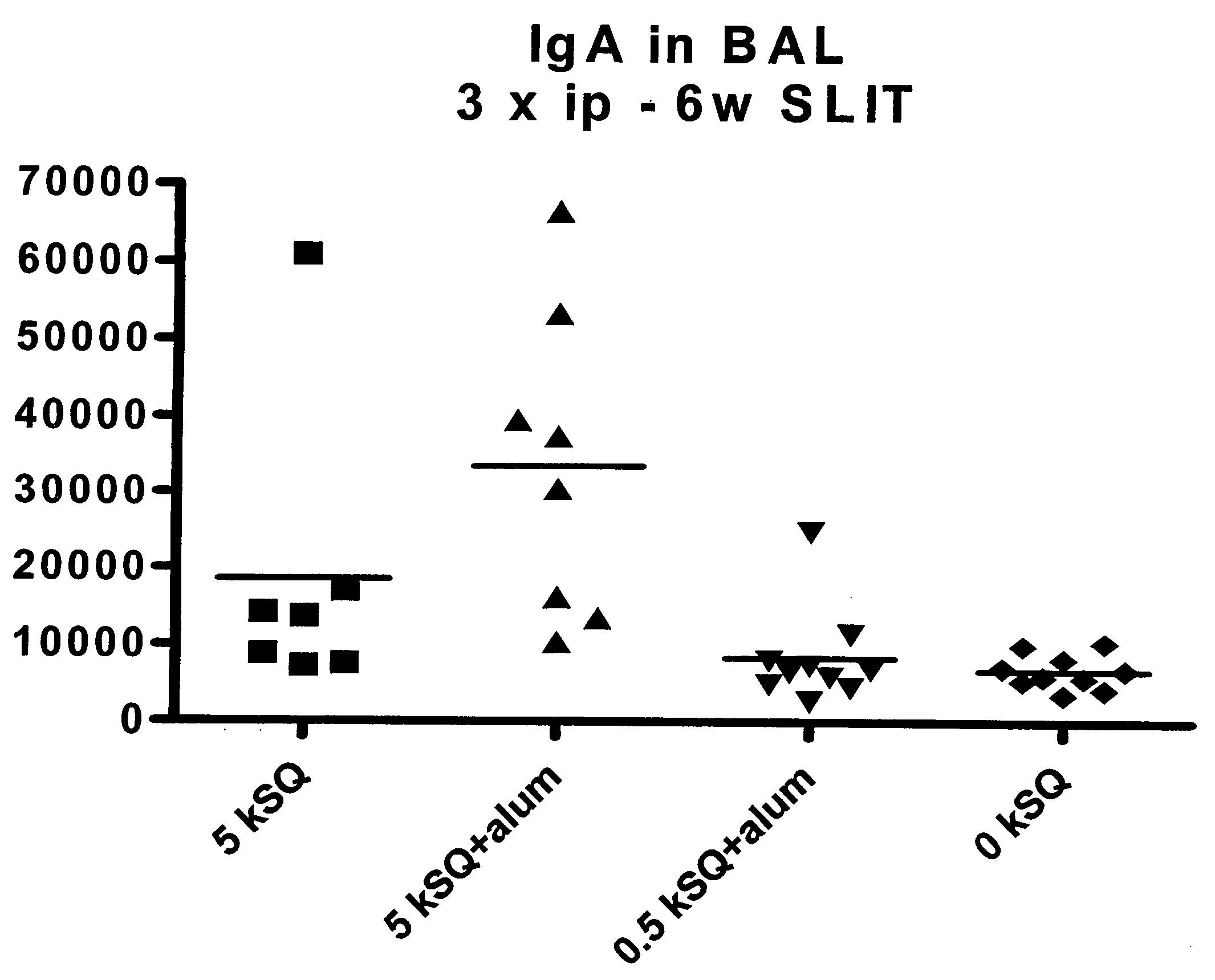
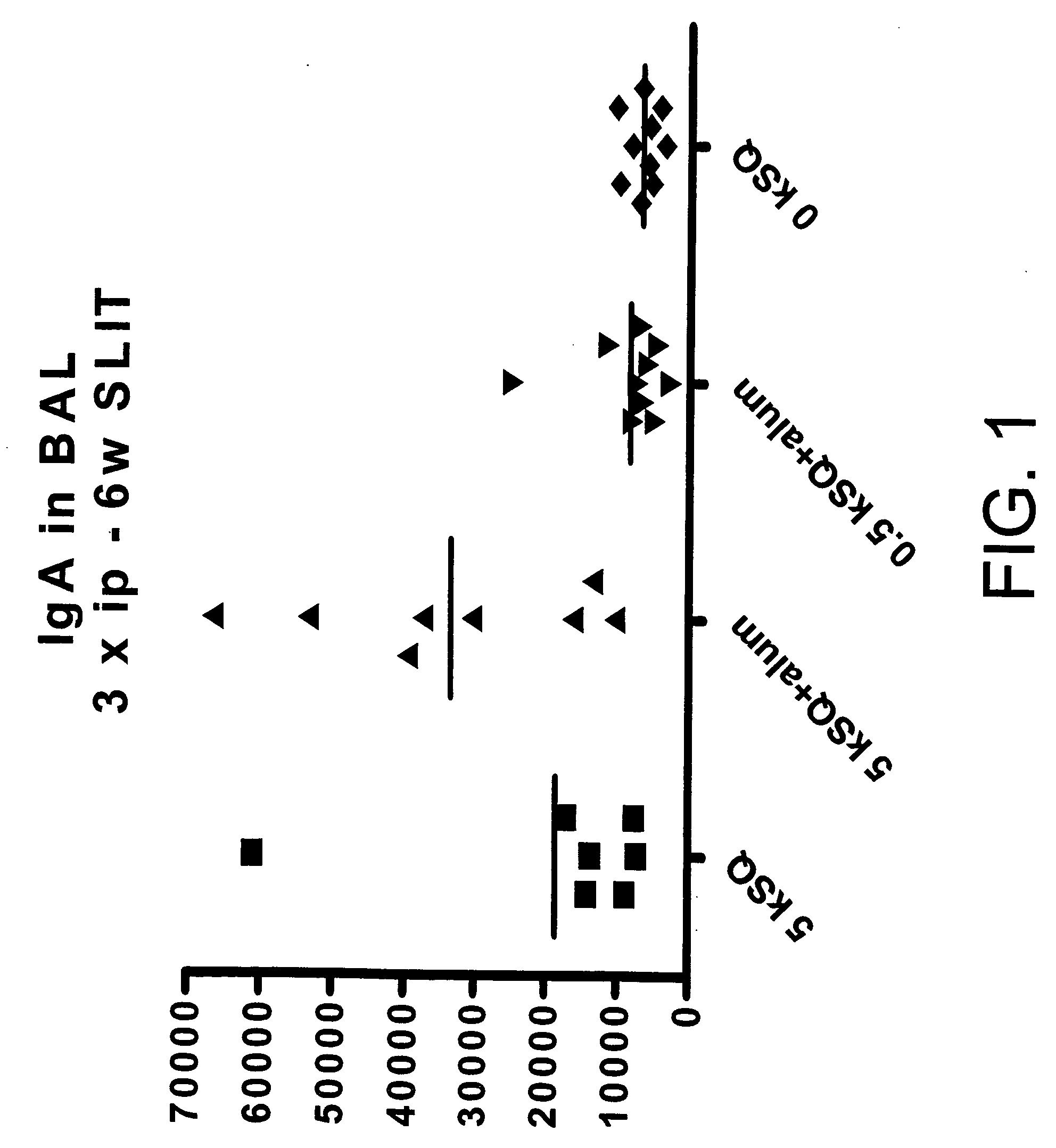
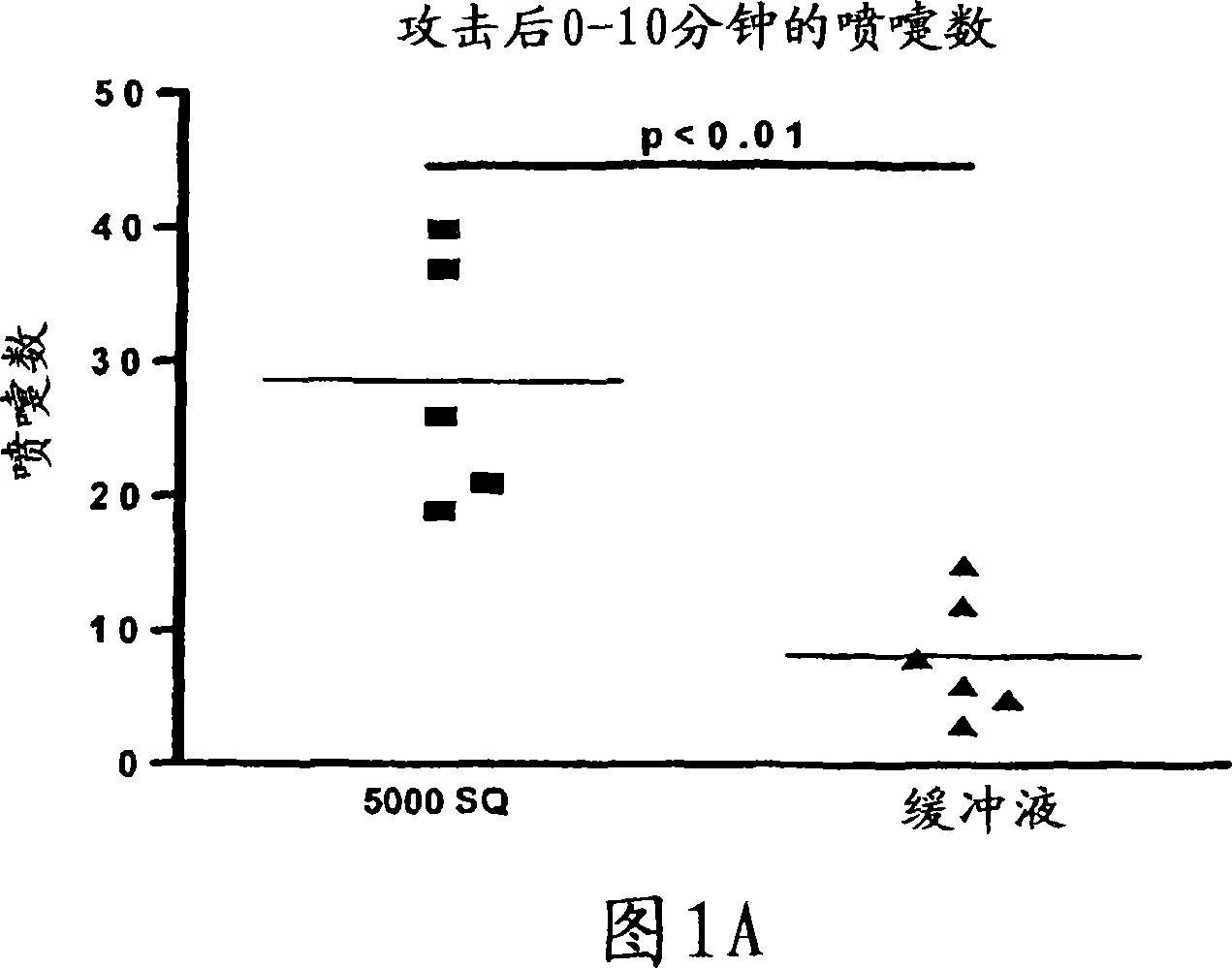
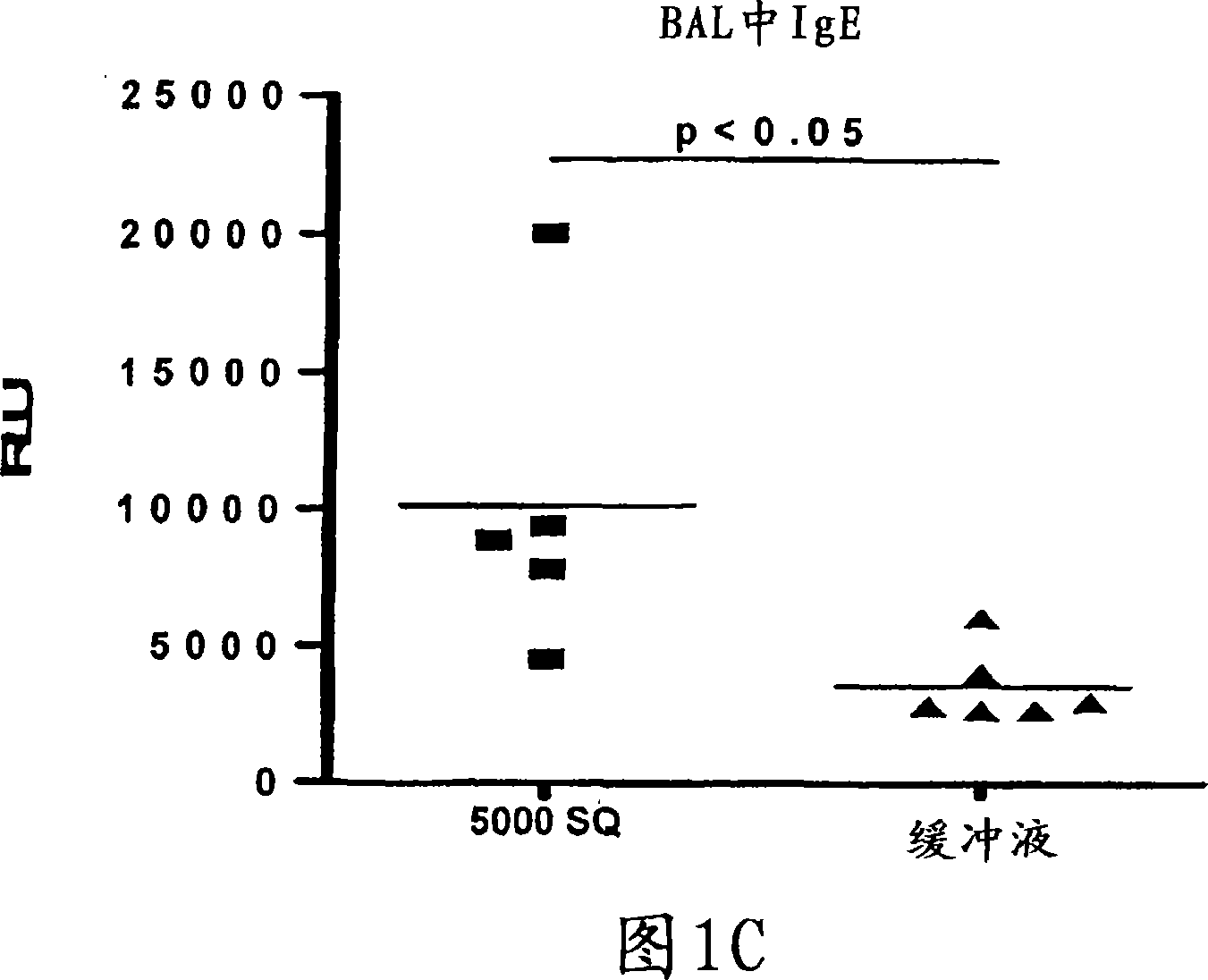
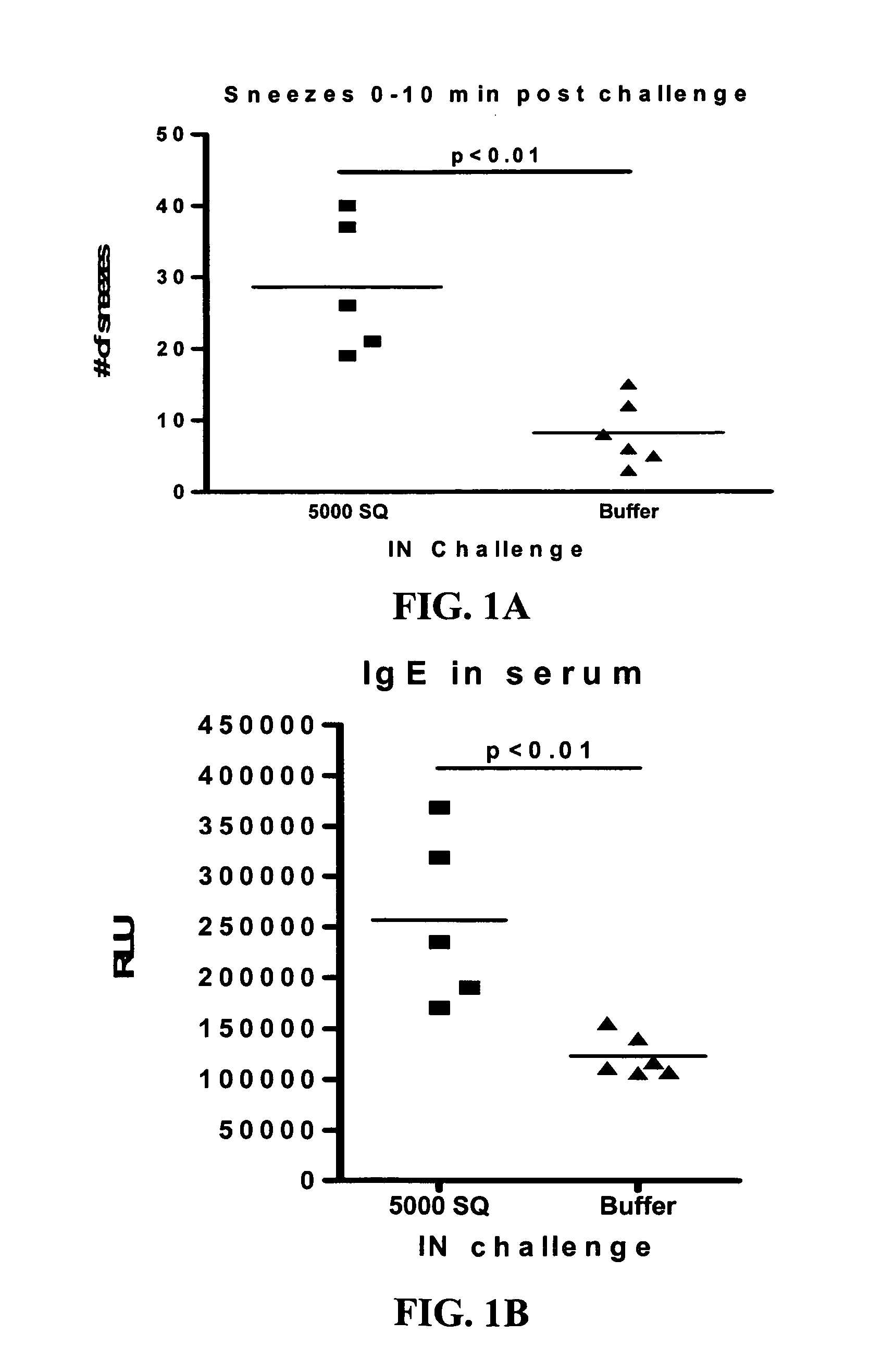
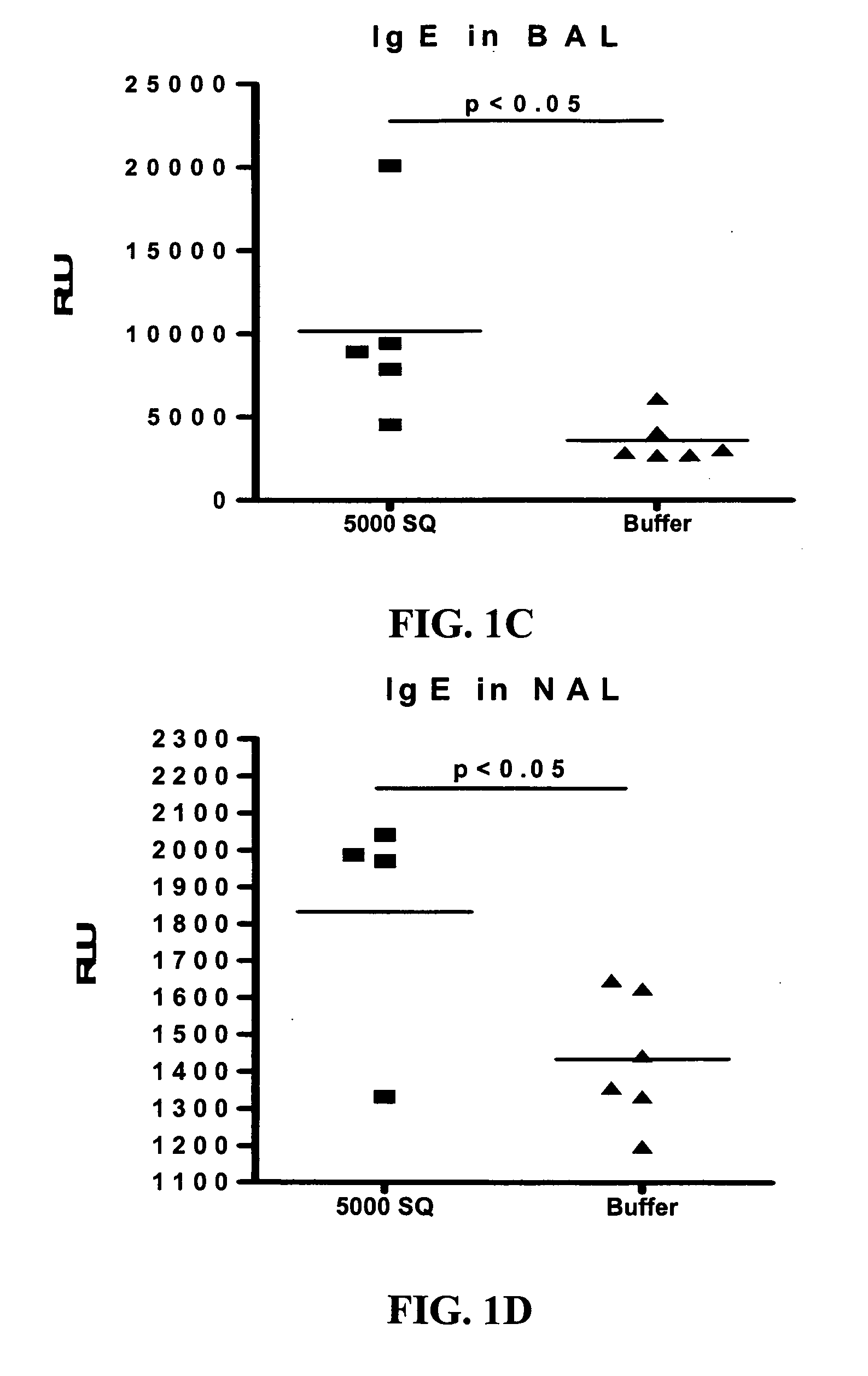
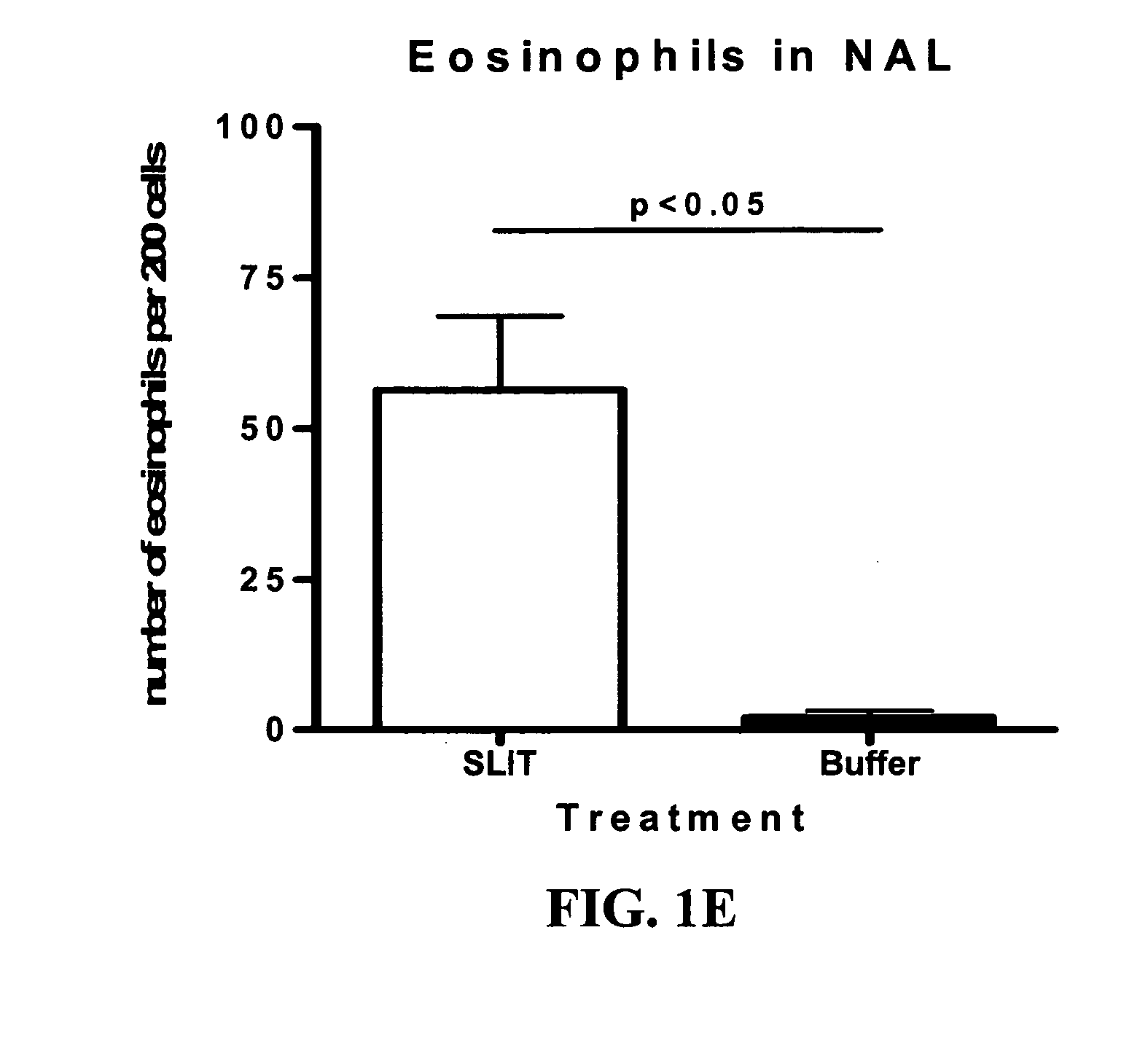
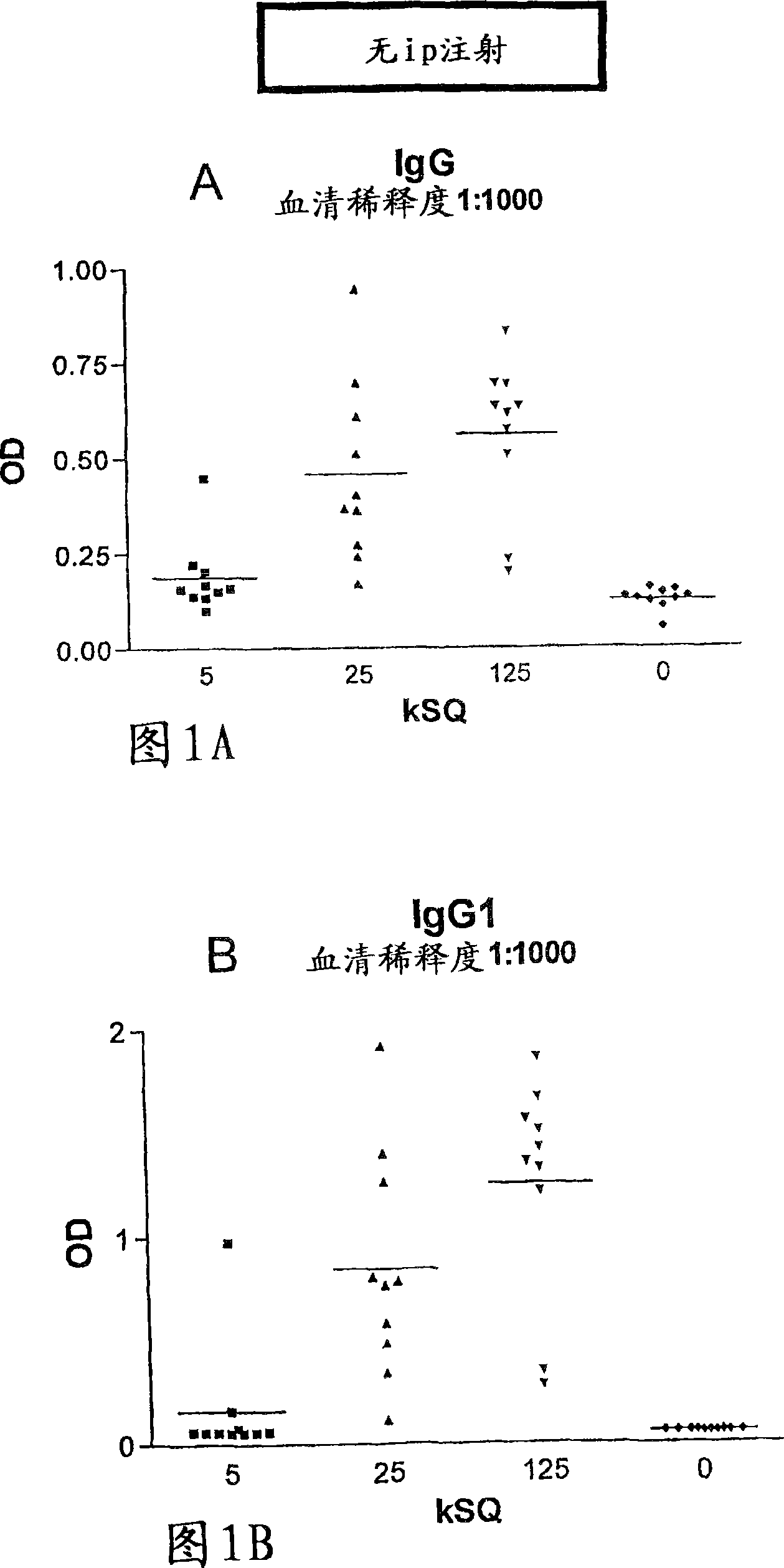
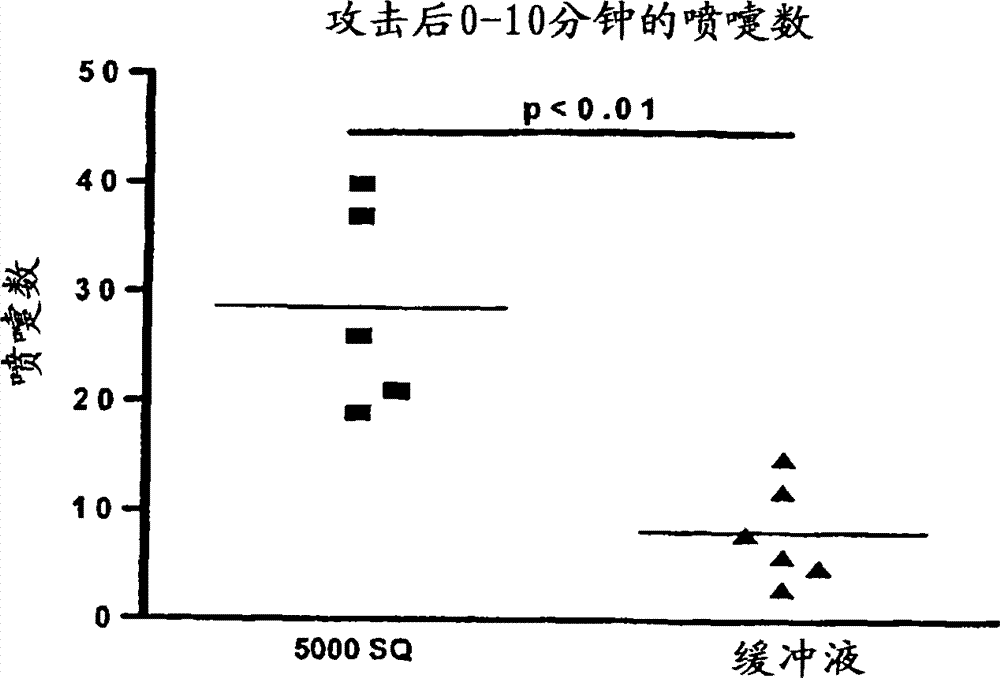
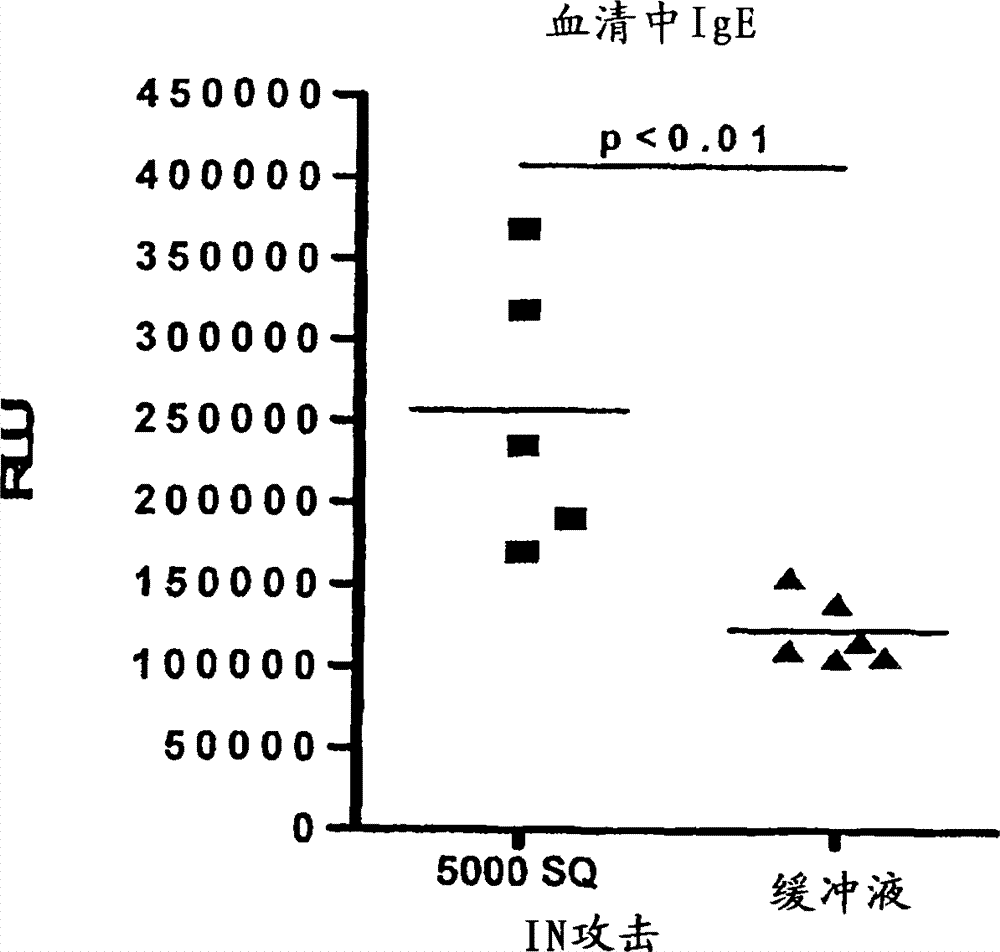
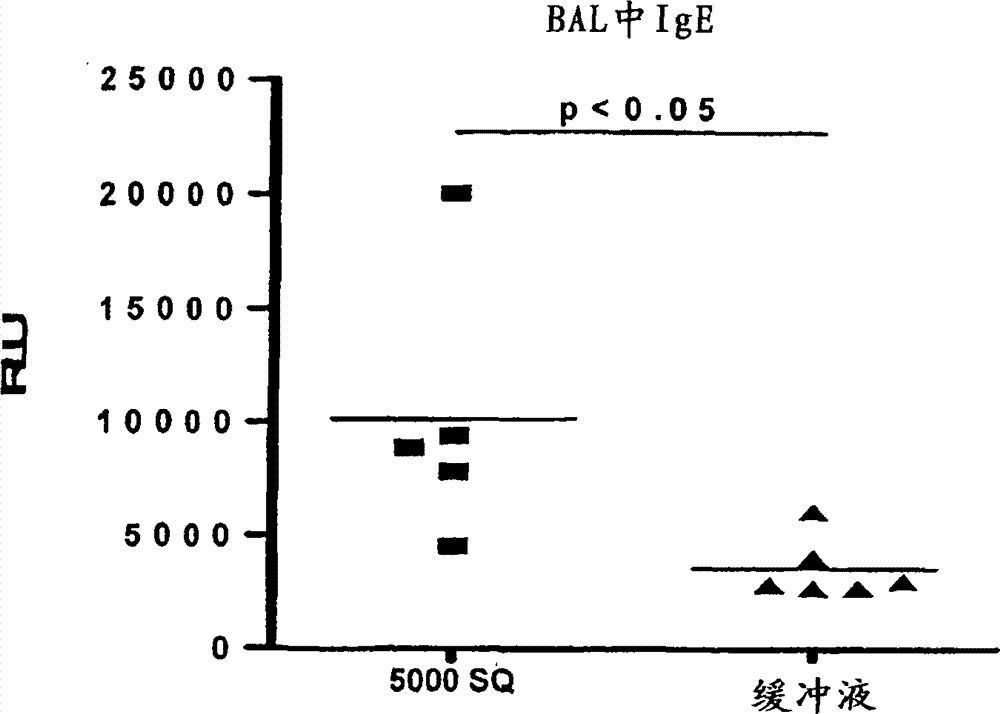
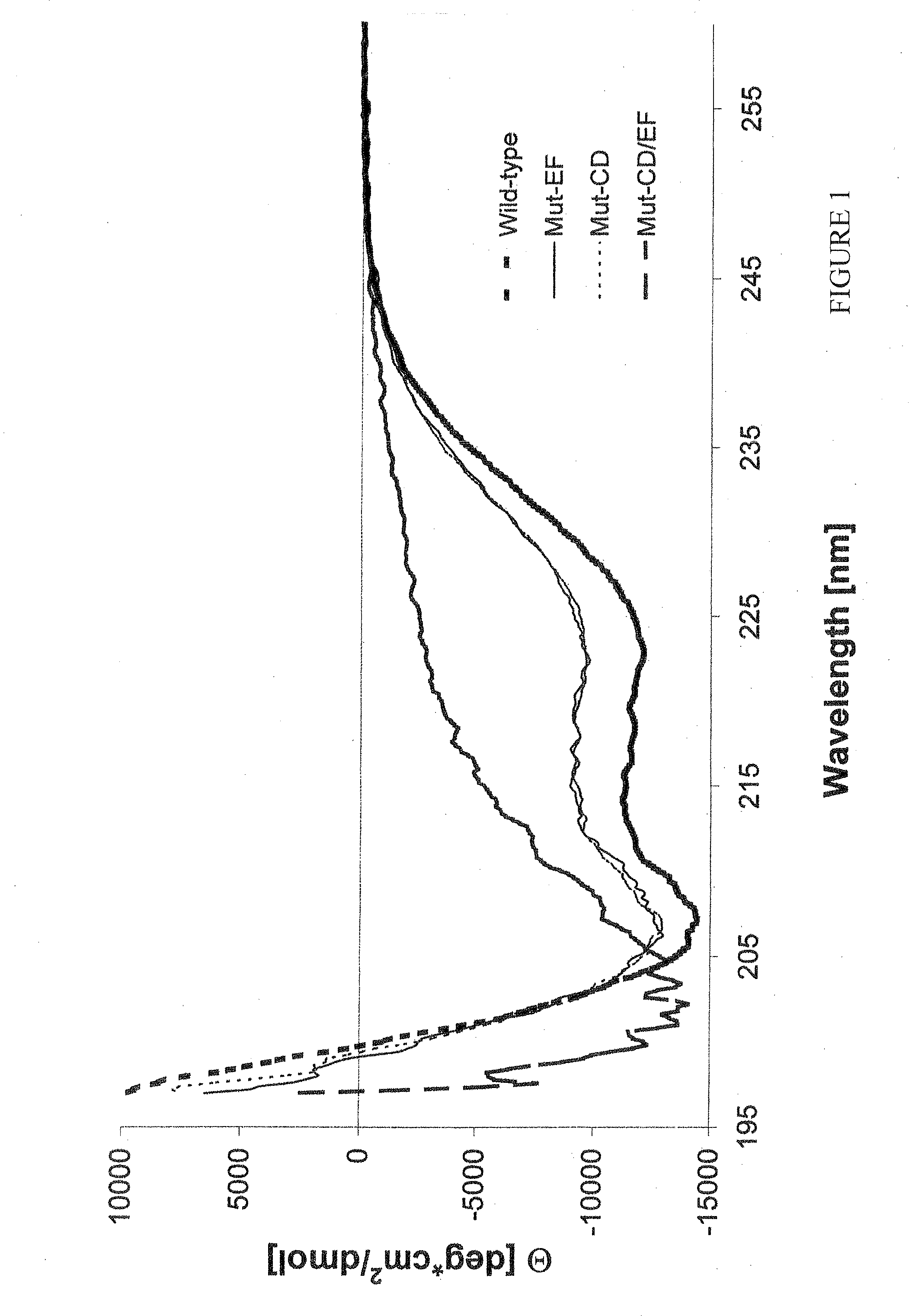
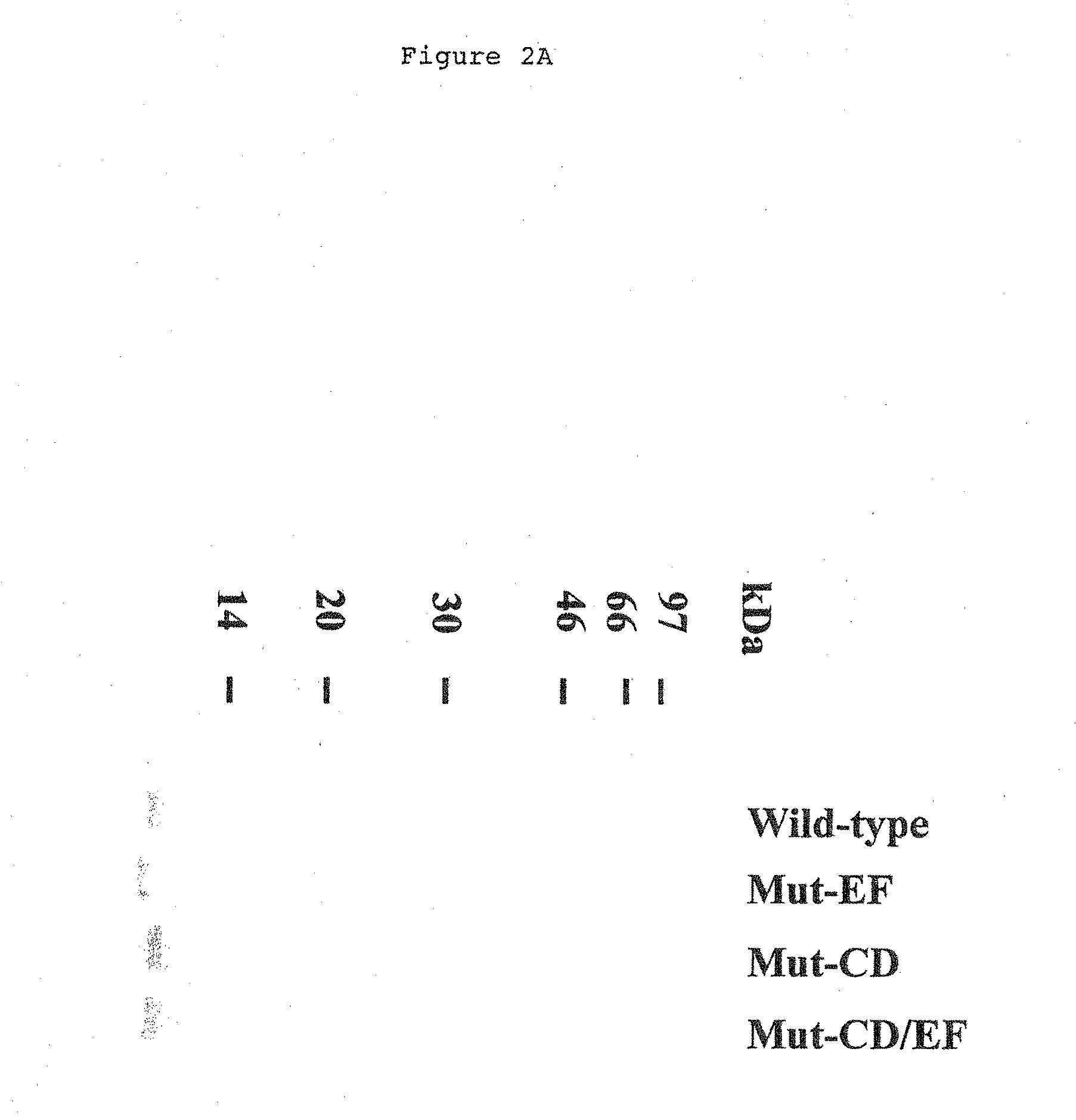
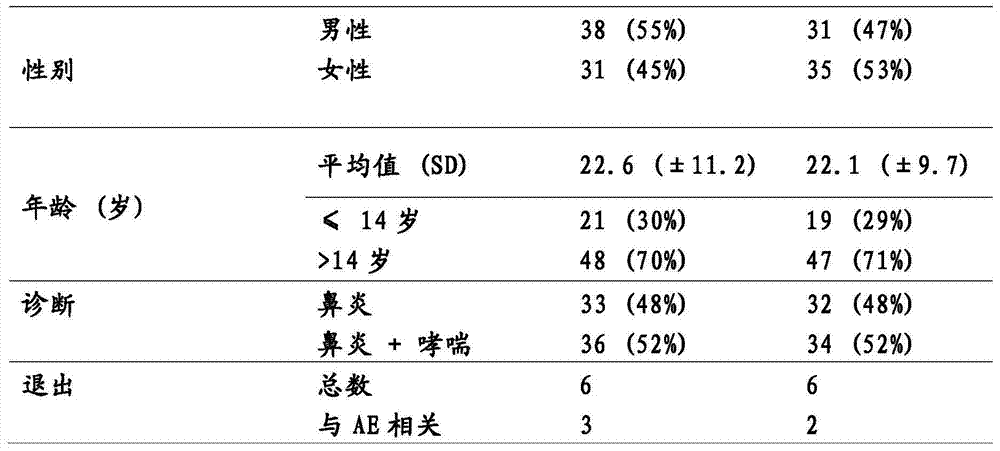
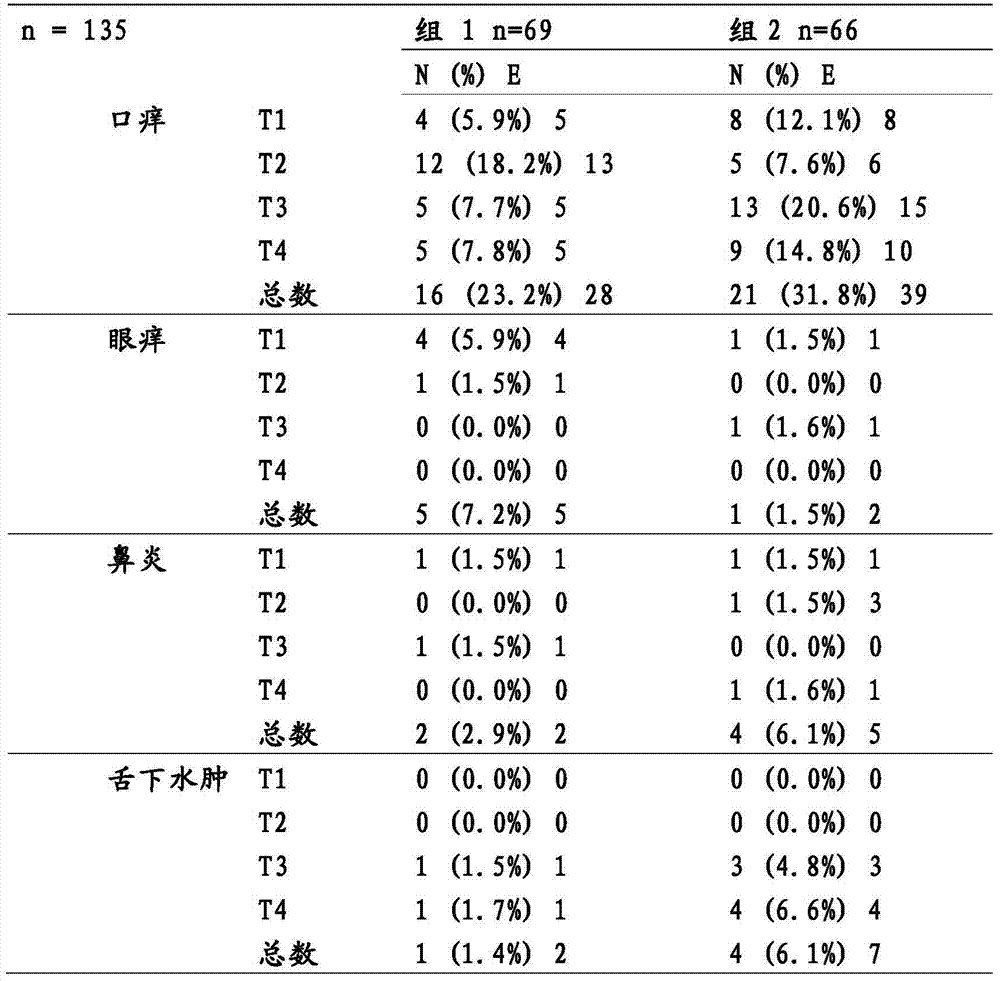
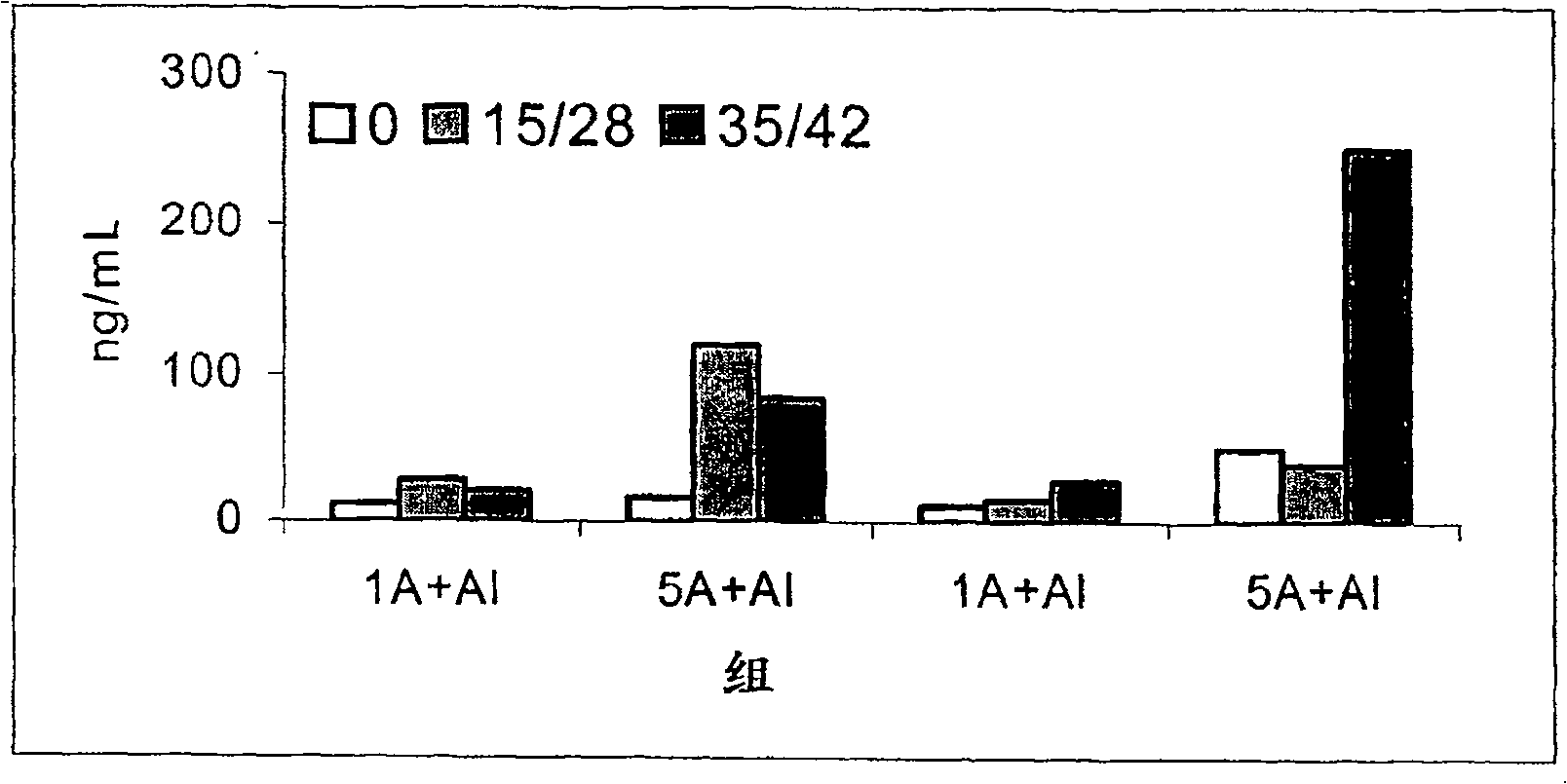
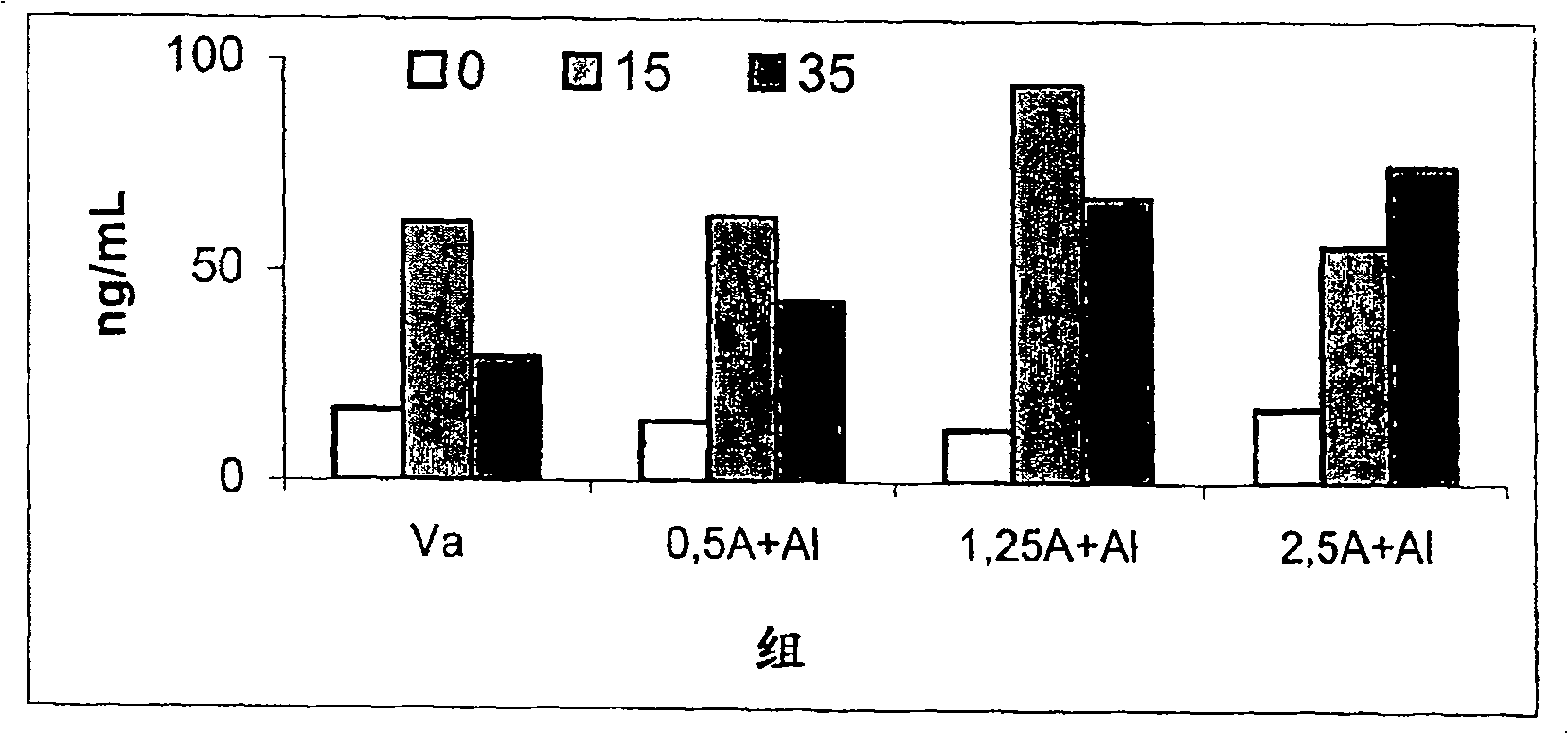
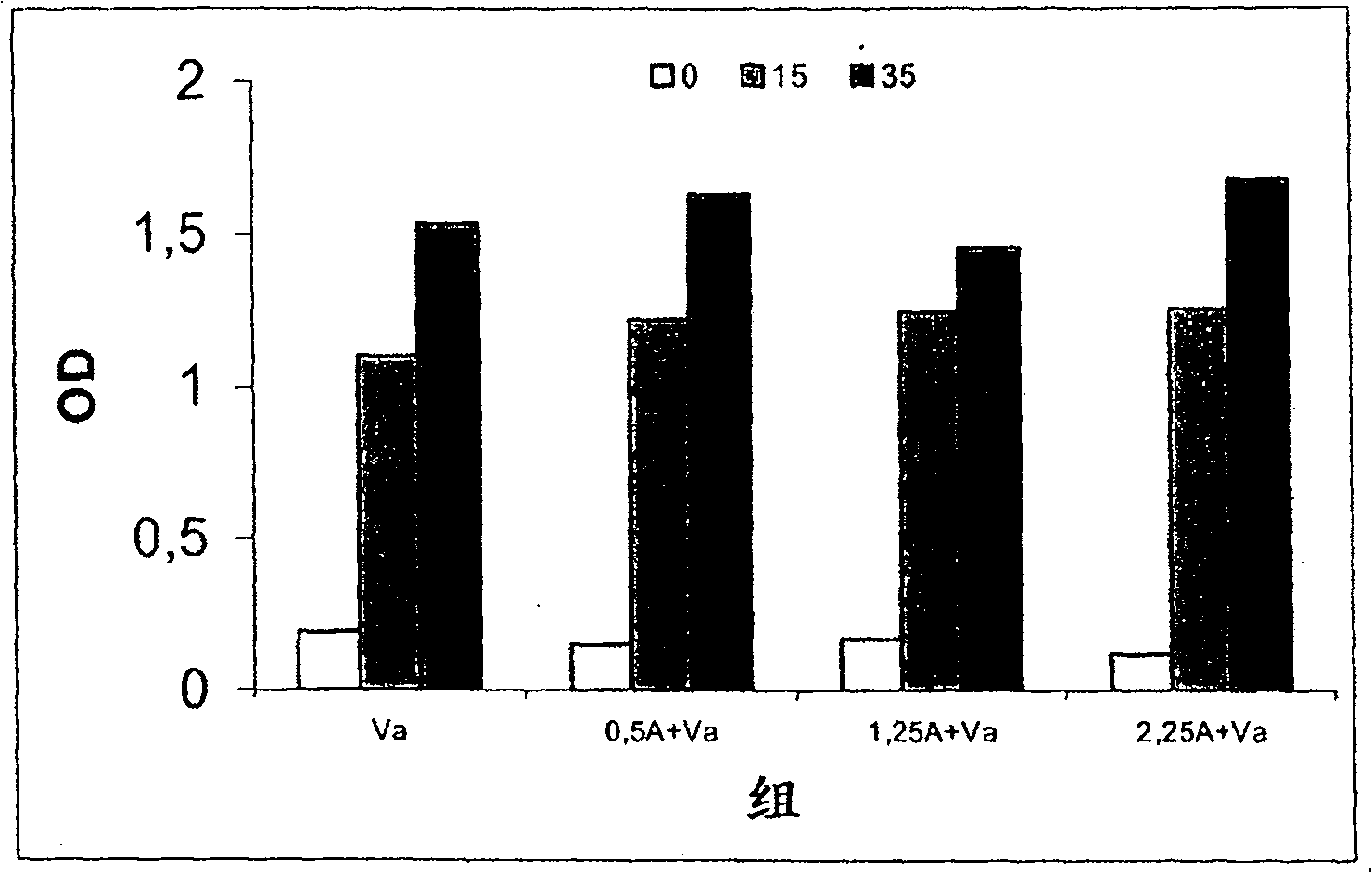
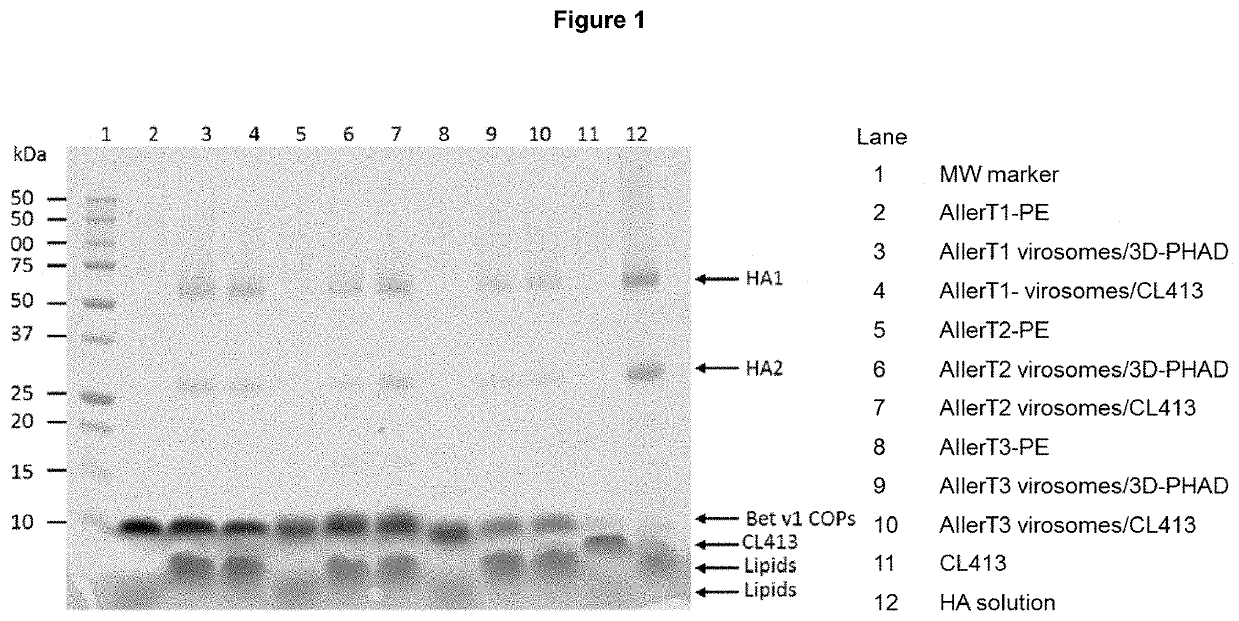
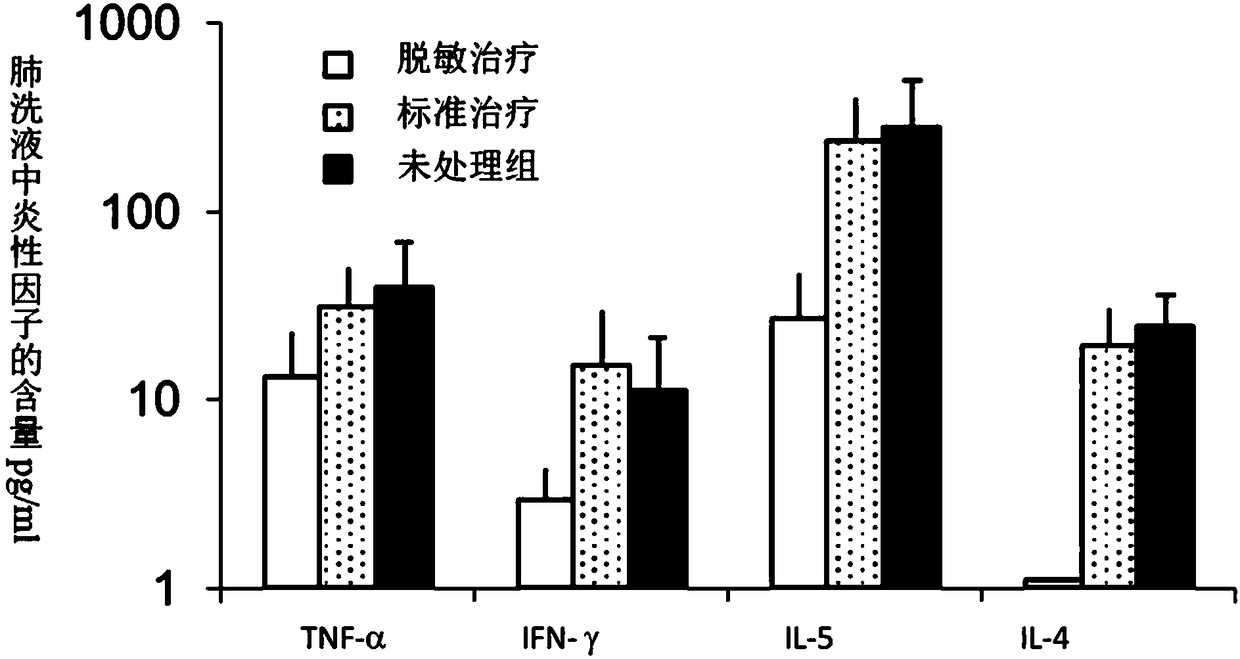
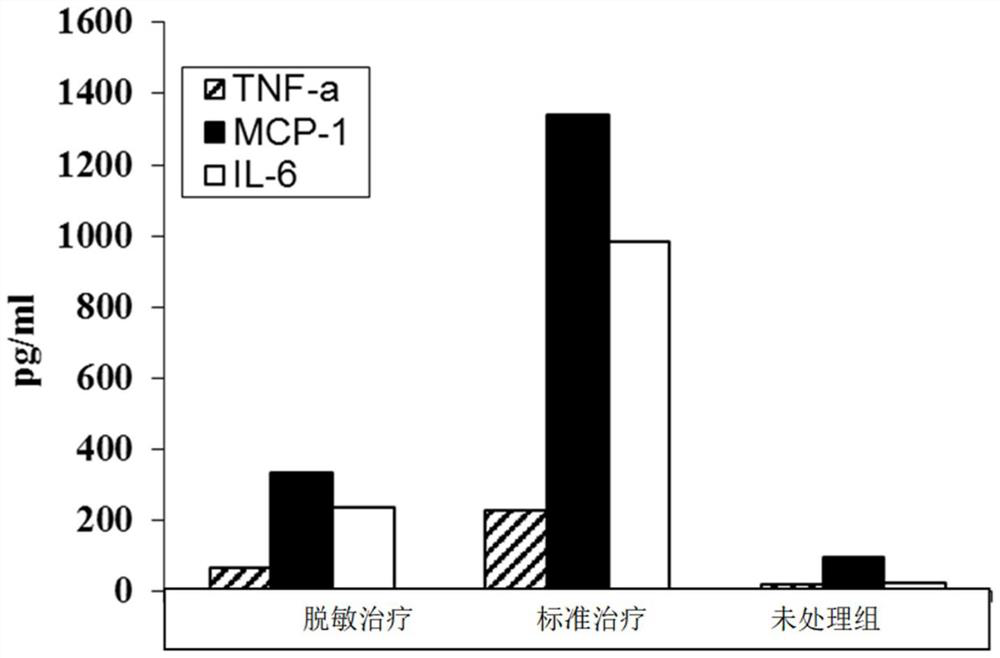
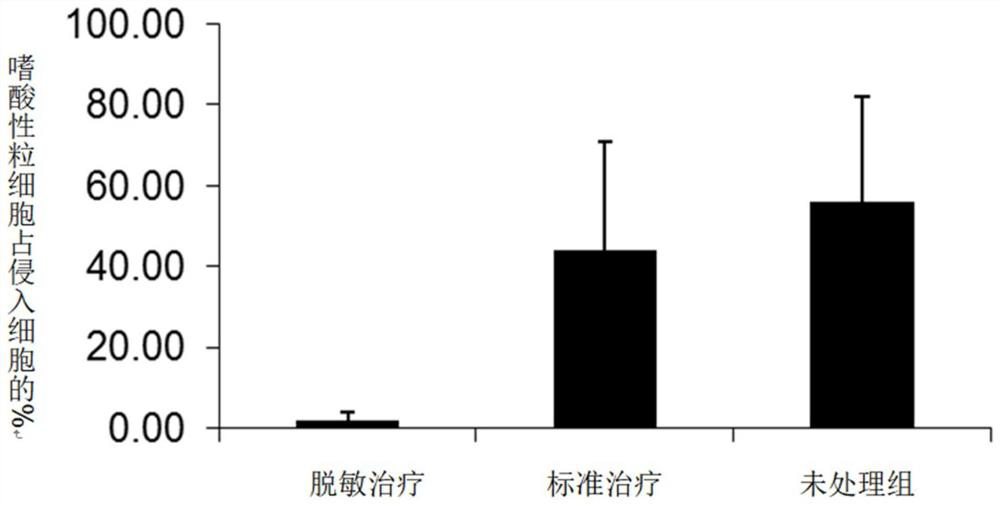
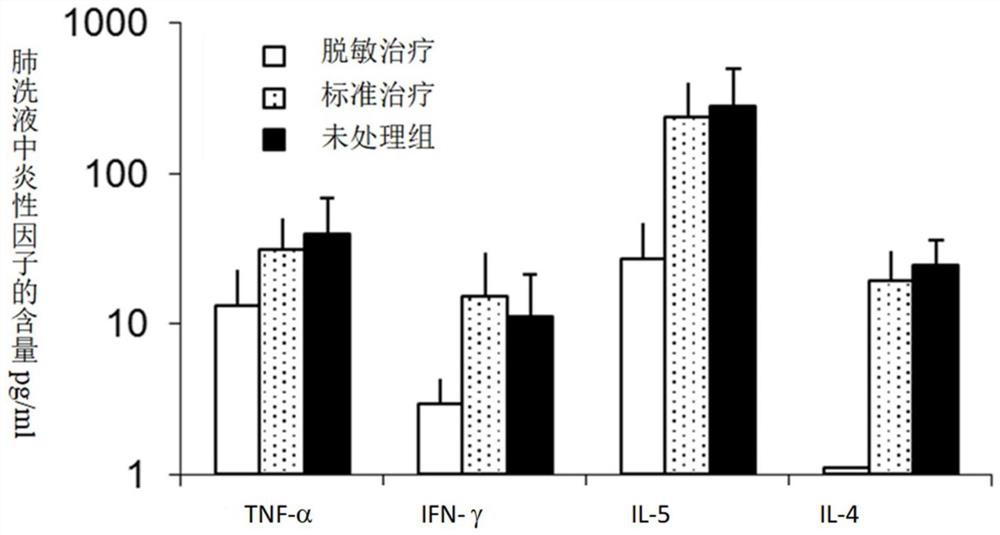
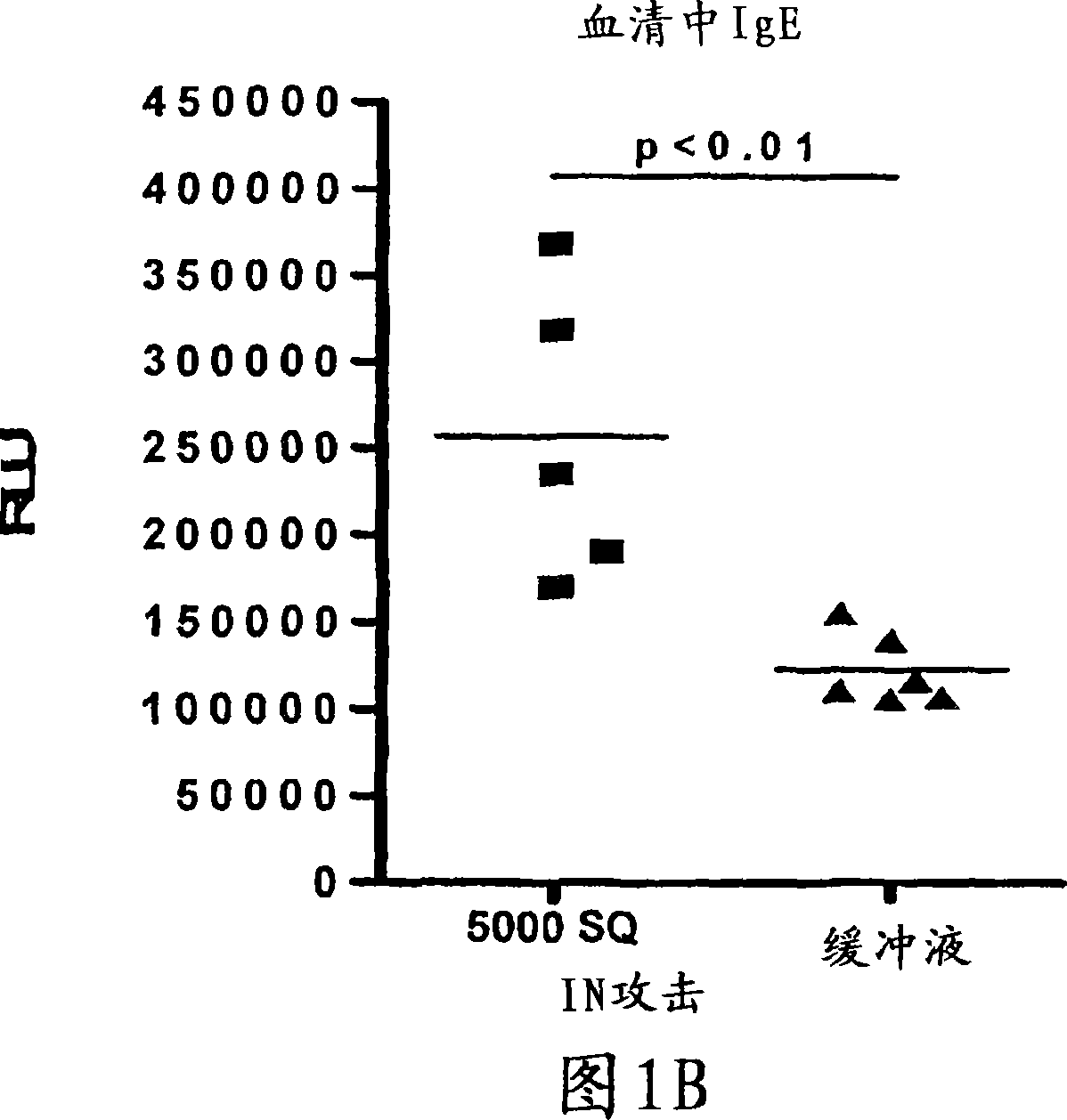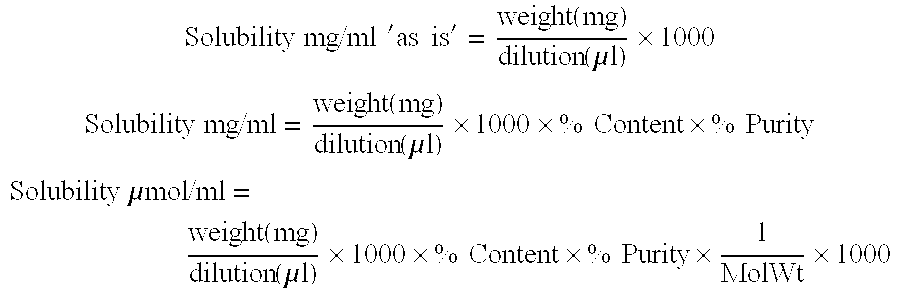Patents
Literature
31 results about "Allergy vaccine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Vaccine allergy. background. Patients receive vaccines to prevent infections and diseases from developing. Although rare, some patients may develop allergic reactions to vaccines. This may occur when an individual is allergic to one or more products contained in the vaccine.
Methods of prevention and treatment of asthma, and allergic conditions
InactiveUS20050013799A1Avoid allergic reactionsEffective preventionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterferon alphaReceptor Inhibition
The present invention relates to allergy vaccines and methods of treating and / or preventing asthma, and allergic conditions. The invention is based on the discovery that inhibiting the ligand / receptor interactions involving, e.g., IgE, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, interferon-alpha, histamine, leukotriene, and their respective receptors, inhibits production of IgE thereby treating or preventing such diseases or conditions. Competitive inhibition of such receptor / ligand interactions is accomplished by immunizing a human or veterinary patient with the interleukin, interferon-alpha, histamine, leukotriene, their receptors, in any combination. Also, the invention relates to inhibiting receptor / ligand interactions involved in IgE production by competitively inhibiting such interactions by administering antibodies to the ligands, receptors, or both, as well as by administering analogs of the receptors (e.g., soluble receptors not associated with a cell).
Owner:ADVANCED BIOTHERAPY
Methods of prevention and treatment of asthama, and allergic conditions
InactiveUS20050013800A1Avoid allergic reactionsEffective preventionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterferon alphaReceptor Inhibition
The present invention relates to allergy vaccines and methods of treating and / or preventing asthma, and allergic conditions. The invention is based on the discovery that inhibiting the ligand / receptor interactions involving, e.g., IgE, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, interferon-alpha, histamine, leukotriene, and their respective receptors, inhibits production of IgE thereby treating or preventing such diseases or conditions. Competitive inhibition of such receptor / ligand interactions is accomplished by immunizing a human or veterinary patient with the interleukin, interferon-alpha, histamine, leukotriene, their receptors, in any combination. Also, the invention relates to inhibiting receptor / ligand interactions involved in IgE production by competitively inhibiting such interactions by administering antibodies to the ligands, receptors, or both, as well as by administering analogs of the receptors (e.g., soluble receptors not associated with a cell).
Owner:ADVANCED BIOTHERAPY
Use of a Liquid Allergy Vaccine Formulation for Oromucosal Administration
InactiveUS20090297564A1Improve treatment adherenceDosage regimen much simplerInorganic non-active ingredientsAllergen ingredientsRegimenOral cavity
Owner:ALK ABELLO SA
Method of preventive treatment of allergy by oromucosal administration of an allergy vaccine
InactiveUS20060171968A1Efficient administrationLittle can be doneSnake antigen ingredientsAllergen ingredientsAllergy preventionProphylactic treatment
The present invention relates to a method of preventive treatment of allergy to an allergen in a subject comprising a) administering an allergy vaccine containing the allergen as active substance to the subject via an oromucosal route, b) wherein the subject to be treated is unsensitised in the sense of exhibiting no lgE response specific to the allergen, c) wherein the subject to be treated is free of clinical symptoms of any allergy, and d) wherein the preventive treatment is aimed at preventing or reducing subsequent clinical symptoms of the allergy associated with the allergen.
Owner:ALK ABELLO SA
Liquid allergy vaccine formulation for oromucosal administration
InactiveUS20060115499A1Good effectFacilitates treatment protocol complianceSenses disorderAllergen ingredientsAdjuvantMetal salts
Use of a composition comprising an allergen and an adjuvant selected from the group consisting of oxygen-containing metal salts for the manufacture of a liquid formulation for preventing or treating allergy in a subject by oromucosal administration, and a method of preventing and treating allergy in a subject by oromucosal administration of the said liquid formulation.
Owner:ALK ABELLO SA
Method of treating allergy by administering an anti-histamine antibody
InactiveUS6846486B1Avoid allergic reactionsEffective preventionPeptide/protein ingredientsImmunoglobulins against cytokines/lymphokines/interferonsInterferon alphaEphA Receptors
The present invention relates to allergy vaccines and methods of treating and / or preventing asthma, and allergic conditions. The invention is based on the discovery that inhibiting the ligand / receptor interactions involving, e.g., IgE, IL-3, IL-4, IL-5, IL-6, IL-10, IL-13, interferon-alpha, histamine, leukotriene, and their respective receptors, inhibits production of IgE thereby treating or preventing such diseases or conditions. Competitive inhibition of such receptor / ligand interactions is accomplished by immunizing a human or veterinary patient with the interleukin, interferon-alpha, histamine, leukotriene, their receptors, in any combination. Also, the invention relates to inhibiting receptor / ligand interactions involved in IgE production by competitively inhibiting such interactions by administering antibodies to the ligands, receptors, or both, as well as by administering analogs of the receptors (e.g., soluble receptors not associated with a cell).
Owner:ADVANCED BIOTHERAPY
Method of preventive treatment of allergy by mucosal administration of an allergy vaccine
InactiveCN101065146ARelieve clinicalReduced characteristicsAllergen ingredientsProphylactic treatmentAnaphylactic reaction
The present invention relates to a method of preventive treatment of allergy to an allergen in a subject comprising administering an allergy vaccine containing the allergen as active substance to a mucosal surface of the subject, a) wherein the subject to be treated is sensitised so as to exhibit an IgE response specific to the allergen, b) wherein the subject to be treated is free of clinical symptoms of the allergy associated with the allergen, and c) wherein the preventive treatment is aimed at preventing or reducing subsequent clinical symptoms of the allergy associated with the allergen.
Owner:ALK ABELLO SA
Method of preventive treatment of allergy by mucosal administration of an allergy vaccine
InactiveUS20060121064A1Avoid symptomsEffective treatmentAllergen ingredientsImmunological disordersAllergy vaccineProphylactic treatment
The present invention relates to a method of preventive treatment of allergy to an allergen in a subject comprising administering an allergy vaccine containing the allergen as active substance to a mucosal surface of the subject, a) wherein the subject to be treated is sensitised so as to exhibit an IgE response specific to the allergen, b) wherein the subject to be treated is free of clinical symptoms of the allergy associated with the allergen, and c) wherein the preventive treatment is aimed at preventing or reducing subsequent clinical symptoms of the allergy associated with the allergen.
Owner:ALK ABELLO SA
Method of preventive treatment of allergy by oromucosal administration of an allergy vaccine
The present invention relates to a method of preventive treatment of allergy to an allergen in a subject comprising a) administering an allergy vaccine containing the allergen as active substance to the subject via an oromucosal route, b) wherein the subject to be treated is unsensitised in the sense of exhibiting no IgE response specific to the allergen, c) wherein the subject to be treated is free of clinical symptoms of any allergy, and d) wherein the preventive treatment is aimed at preventing or reducing subsequent clinical symptoms of the allergy associated with the allergen.
Owner:ALK ABELLO SA
Liquid allergy vaccine formulation for oromucosal administration
InactiveCN101048176AImprove adaptabilityAccurate dosageSenses disorderPharmaceutical delivery mechanismAdjuvantBuccal administration
Use of a composition comprising an allergen and an adjuvant selected from the group consisting of oxygen-containing metal salts for the manufacture of a liquid formulation for preventing or treating allergy in a subject by oromucosal administration, and a method of preventing and treating allergy in a subject by oromucosal administration of the said liquid formulation.
Owner:ALK ABELLO SA
Use of a liquid allergy vaccine formulation for oromucosal administration
InactiveCN101340892AImprove treatment complianceSimple dosing regimenInorganic non-active ingredientsAllergen ingredientsRegimenBuccal administration
Owner:ALK ABELLO SA
Allergy vaccine composition
ActiveUS20180147235A1Induce immune toleranceImprove the quality of lifeOrganic active ingredientsBacterial antigen ingredientsBacteroidesAcidocella
The present invention provides an allergy vaccine composition that is useful as a prophylactic or therapeutic agent for an allergic disease and can safely and effectively induce immune tolerance. The vaccine composition is to be administered to a human or animal with an allergic disease for the prevention or treatment of the allergic disease. The vaccine composition contains: an immunomodulator which is a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt of the lipopolysaccharide; and at least one allergen.
Owner:NITTO DENKO CORP
Use of an adjuvanted allergy vaccine formulation for parenteral administration
The present invention relates to the use of a seasonal allergen composition for the manufacture of a vaccine formulation for preventing or treating allergy to the allergen composition in a subject by parenteral administration, wherein the vaccine formulation is administered in a dosage regimen comprising an up-dosing phase, wherein the up-dosing phase partly or wholly overlaps with the allergen season of the allergen composition.
Owner:ALK ABELLO SA
Allergy vaccine composition for mucosal administration
InactiveUS20100291155A1Optimise vivo concentrationEasy to useAllergen ingredientsImmunological disordersAdjuvantActive state
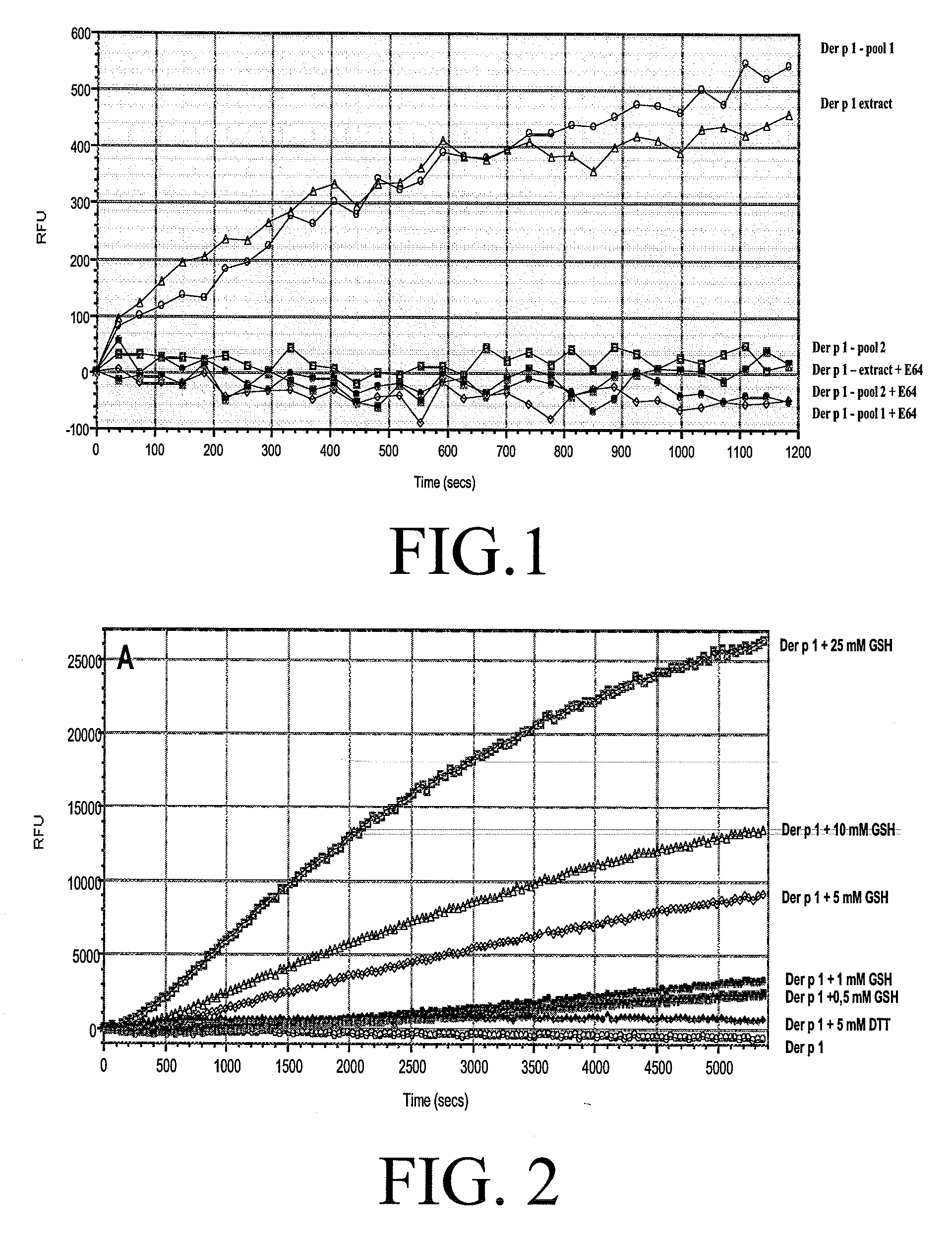
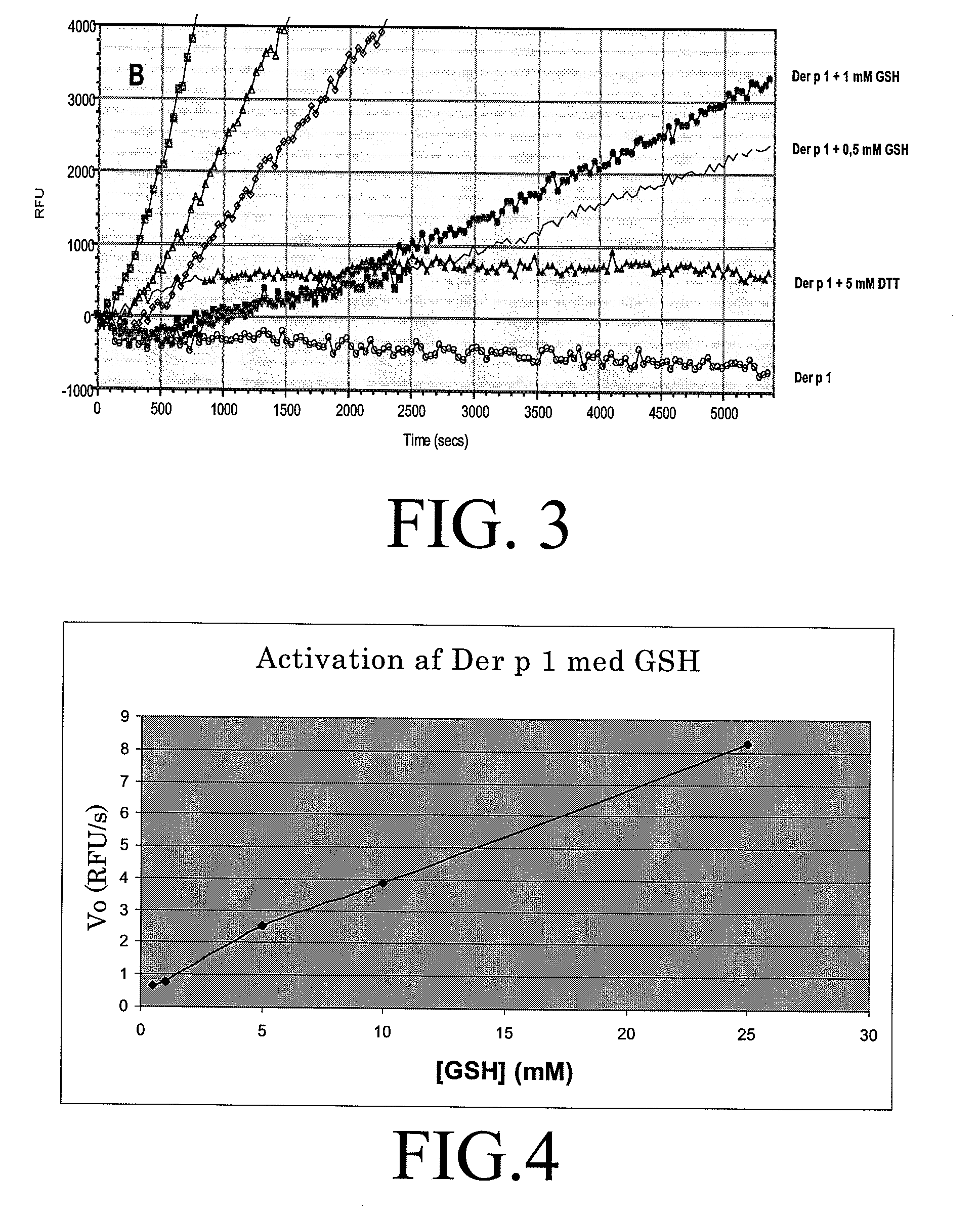
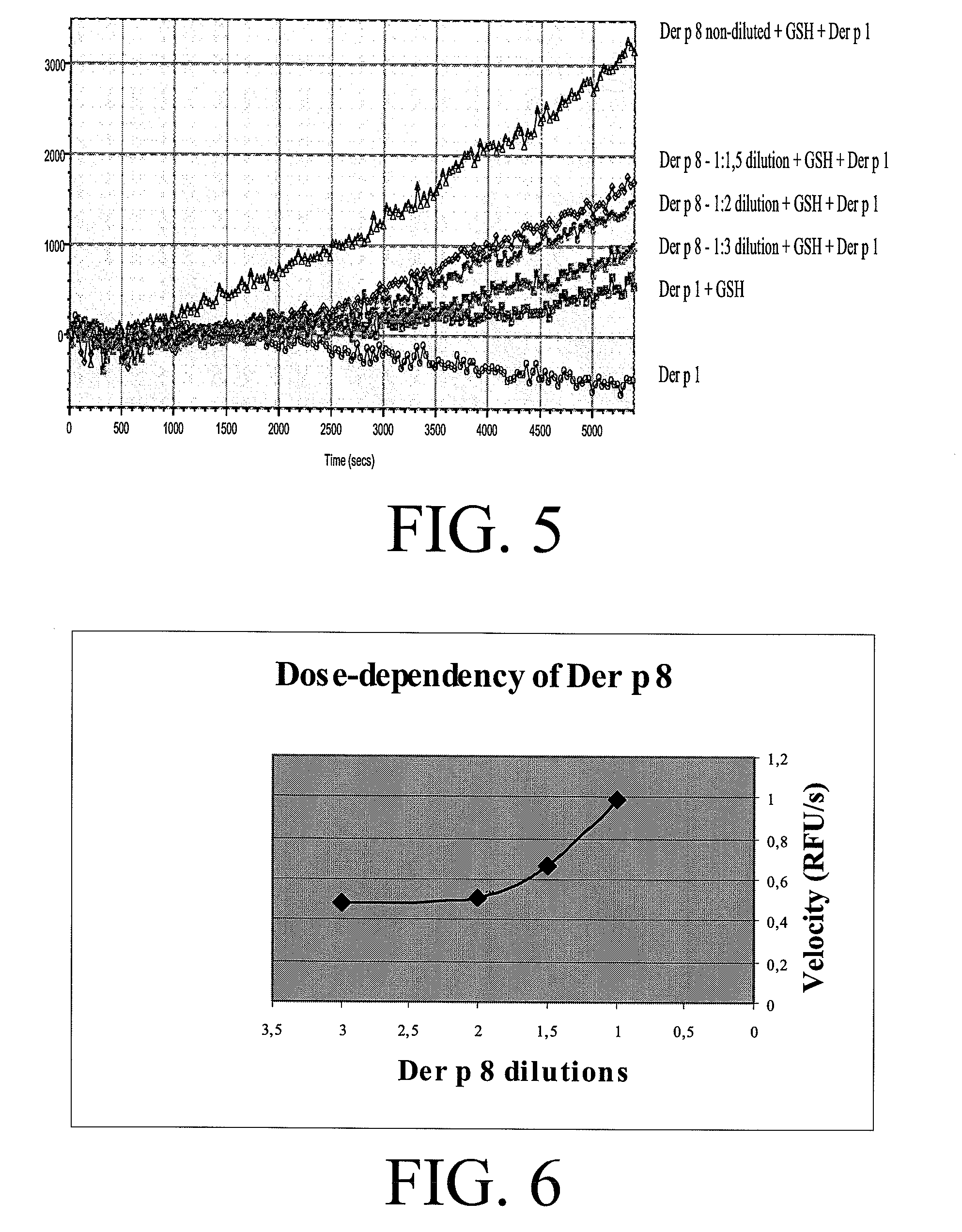
The present invention relates to an allergy vaccine composition for mucosal administration comprising a cysteine protease allergen in a reduced active state or in an oxidised inactive state, The inventions further relates to an adjuvant system for use in a vaccine for mucosal administration comprising a cysteine protease.
Owner:ALK ABELLO SA
Method of preventive treatment of allergy by mucosal administration of an allergy vaccine
InactiveCN101065146BRelieve clinicalReduced characteristicsAllergen ingredientsAnaphylaxisProphylactic treatment
The present invention relates to a method of preventive treatment of allergy to an allergen in a subject comprising administering an allergy vaccine containing the allergen as active substance to a mucosal surface of the subject, a) wherein the subject to be treated is sensitised so as to exhibit an IgE response specific to the allergen, b) wherein the subject to be treated is free of clinical symptoms of the allergy associated with the allergen, and c) wherein the preventive treatment is aimed at preventing or reducing subsequent clinical symptoms of the allergy associated with the allergen.
Owner:ALK ABELLO SA
Hypoallergenic Mutant Polypeptides Based on Fish Parvalbumin
InactiveUS20100216717A1Less allergenic activitySugar derivativesPeptide/protein ingredientsCarpProphylactic vaccination
The present invention relates to non-naturally occurring polypeptides derived from fish allergens such as parvalbumin Cyp c 1.01 from carp. The polypeptides display reduced allergenic activity and are useful as allergy vaccines for treatment of sensitized allergic patients and for prophylactic vaccination.
Owner:BIOMAY PROD & HANDELS GMBH
Use of a liquid allergy vaccine formulation for oromucosal administration
InactiveCN103933562AImprove treatment complianceSimple dosing regimenInorganic non-active ingredientsAllergen ingredientsDosing regimenBuccal administration
The present invention relates to the use of an allergen for the manufacture of a liquid vaccine formulation for preventing or treating allergy in a subject by oromucosal administration in a dosing regimen comprising no progressive dosage.
Owner:ALK ABELLO SA
Peptides for vaccine against birch allergy
ActiveUS20130243798A1Wide coverageBroad utilityCompound screeningSenses disorderAllergyOptimal combination
Owner:CIRCASSIA
Pharmaceutical product for up-dosing of allergy vaccine
This invention relates to the use of a pharmaceutical product comprising allergen and optionally an adjuvant for fast up-dosing in connection with allergy vaccination wherein a reduced number of injections are used. The invention also relates to the pharmaceutical product as such.
Owner:ALK ABELLO SA
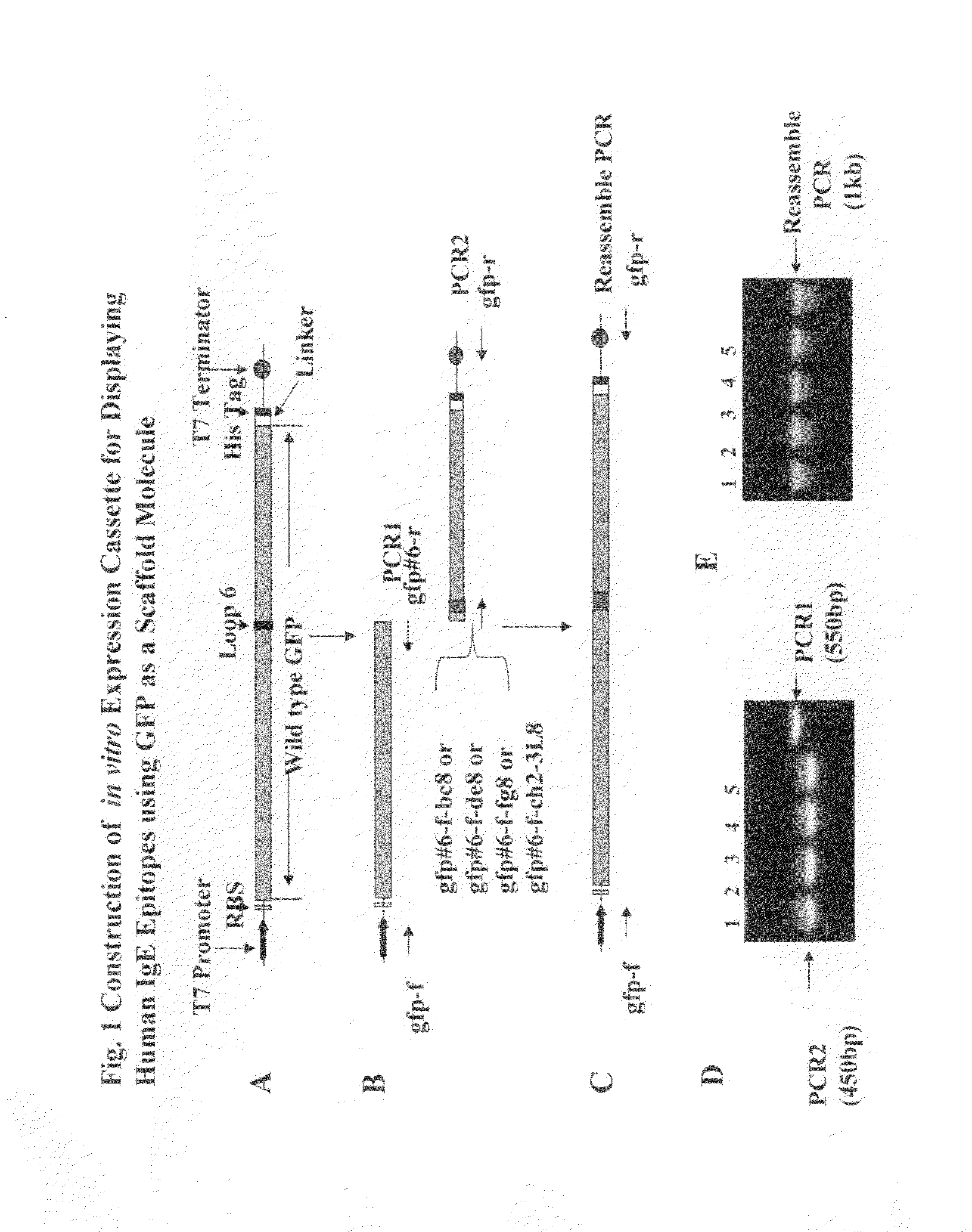
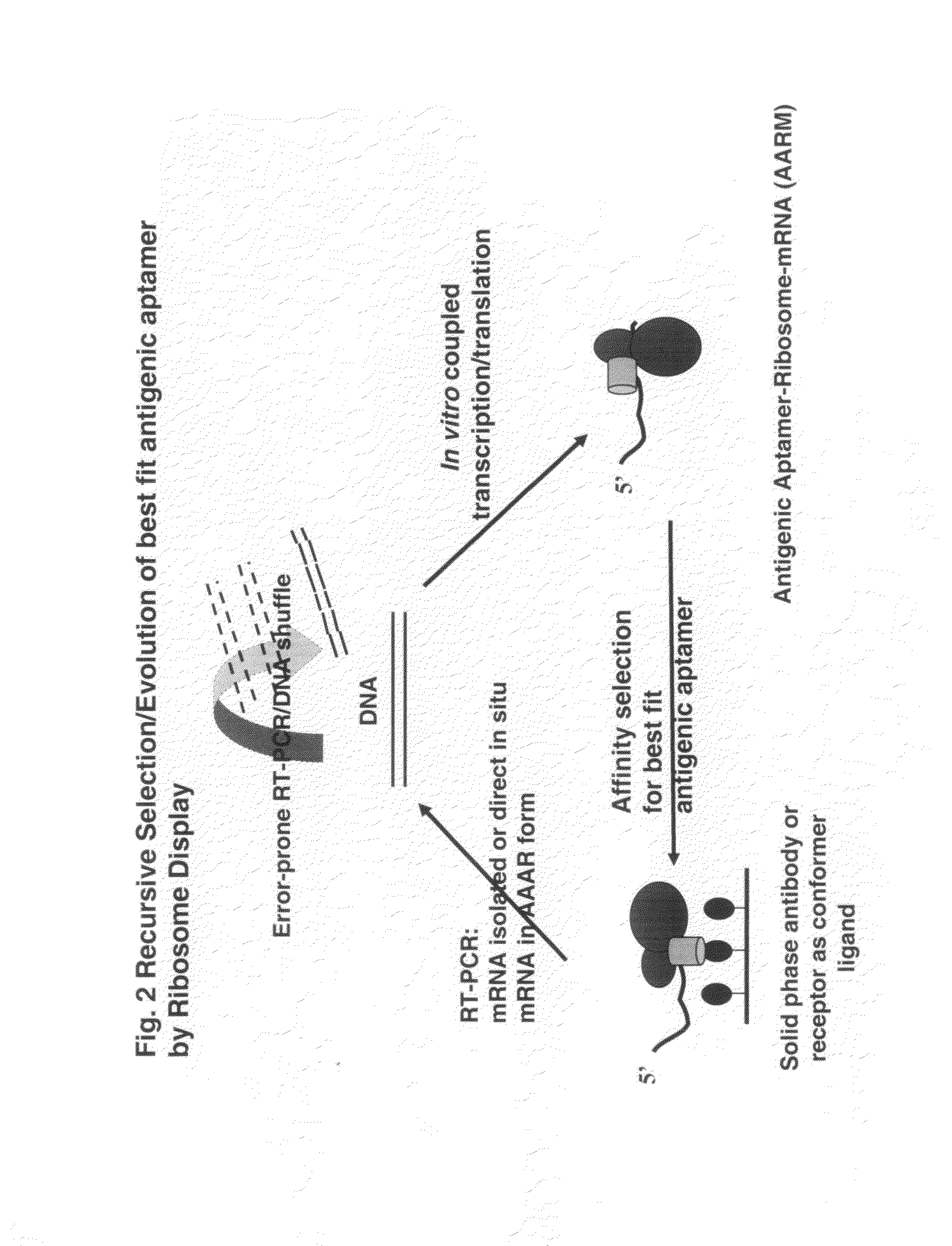
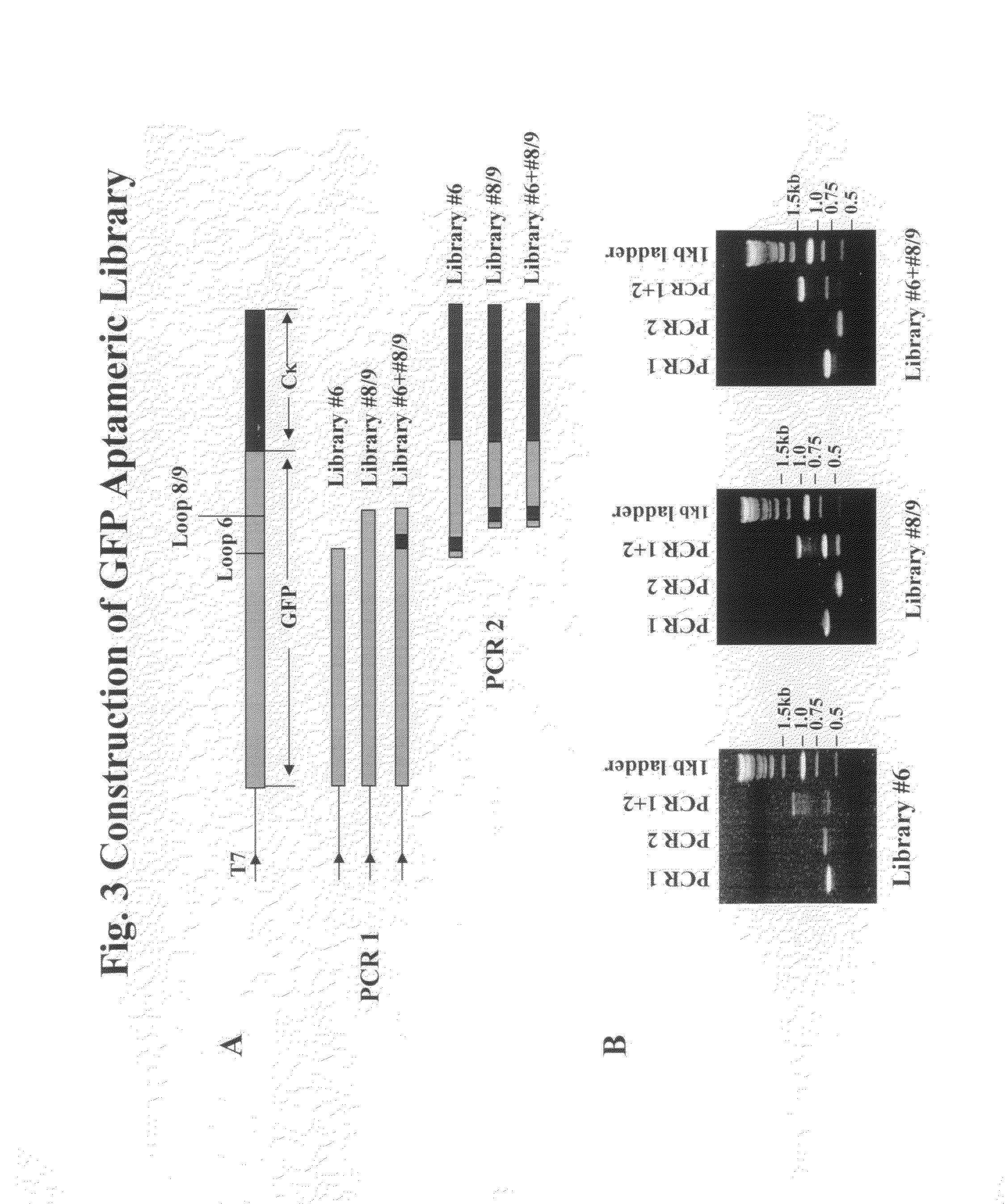
Aptameric IgE peptides in a protein scaffold as an allergy vaccine
ActiveUS8865179B2Reduce physical stressEnhances viability of assaying immunogenicityImmunoglobulin superfamilyAntibody mimetics/scaffoldsFc(alpha) receptorADAMTS Proteins
A method is disclosed wherein an antigenic B-cell epitope is discovered by in vitro transcription and translation of pre-determined sequence on thermostable protein scaffolds detected by antibodies to a native protein. Immune reactivities of IgE B-cell epitopes from pre-determined IgE sequences from the constant region, engaged in binding to high affinity IgE Fc receptors, placed in a loop of green fluorescent protein (GFP) were shown immuno-reactive with anti-IgE. Moreover, the antigenic B-cell epitope can be further optimized by molecular evolution, selected by conformer antibody and receptor on solid phase via ribosome display. Alternatively, a random aptameric antigenic sequence inserted into a loop of the protein scaffold, mimicking a pre-determined sequence of a native protein, can be selected by solid phase conformer antibody and receptor via ribosome display. High affinity binding of B-cell epitopes selected from the random aptameric library with antibodies to native pre-determined B-cell epitope of a native protein can be optimized by molecular evolution with error-prone RT-PCR by ribosome display.
Owner:SWEY SHEN ALEXCHEN
Liquid allergy vaccine formulation for oromucosal administration
InactiveCN101048176BImprove adaptabilityAccurate dosageSenses disorderAllergen ingredientsAdjuvantAllergic reaction
Use of a composition comprising an allergen and an adjuvant selected from the group consisting of oxygen-containing metal salts for the manufacture of a liquid formulation for preventing or treating allergy in a subject by oromucosal administration, and a method of preventing and treating allergy in a subject by oromucosal administration of the said liquid formulation.
Owner:ALK ABELLO SA
Allergy vaccine composition, production method thereof and use of same in allergy treatment
InactiveCN100540052CEffective treatmentAvoid generatingPeptide/protein ingredientsAllergen ingredientsAdjuvantAllergy treatments
The present invention relates to the field of immunology, immuno-allergy and especially the use of adjuvants or carriers for modulating the immune response to allergens. The object of the present invention is to prepare a therapeutic or prophylactic pharmaceutical preparation using bacterial proteoliposomes which, when applied to allergic individuals, is able to switch Th2 and IgE dependent allergic responses into protective Th1 responses ; and it also prevents the emergence and development of allergies in still non-allergic individuals. The vaccine composition comprises proteoliposomes from Gram-negative bacteria coupled to an allergen and optionally other adjuvants or antigens. The present invention also relates to methods of preparing the inventive vaccine composition and a two-dose immunization regimen with said vaccine composition.
Owner:INST FINLAY CENT DE INVESTIGACION PRODUCCION DE VACUNAS Y SUEROS +1
Allergy vaccine composition
ActiveUS10688120B2Induce immune toleranceEasy to manageOrganic active ingredientsBacterial antigen ingredientsDiseaseImmunomodulating Agent
The present invention provides an allergy vaccine composition that is useful as a prophylactic or therapeutic agent for an allergic disease and can safely and effectively induce immune tolerance. The vaccine composition is to be administered to a human or animal with an allergic disease for the prevention or treatment of the allergic disease. The vaccine composition contains: an immunomodulator which is a lipopolysaccharide derived from at least one gram-negative bacterium selected from the group consisting of Serratia, Leclercia, Rahnella, Acidicaldus, Acidiphilium, Acidisphaera, Acidocella, Acidomonas, Asaia, Belnapia, Craurococcus, Gluconacetobacter, Gluconobacter, Kozakia, Leahibacter, Muricoccus, Neoasaia, Oleomonas, Paracraurococcus, Rhodopila, Roseococcus, Rubritepida, Saccharibacter, Stella, Swaminathania, Teichococcus, Zavarzinia, Pseudomonas, Achromobacter, Bacillus, Methanoculleus, Methanosarcina, Clostridium, Micrococcus, Flavobacterium, Pantoea, Acetobacter, Zymomonas, Xanthomonas, and Enterobacter, or a salt of the lipopolysaccharide; and at least one allergen.
Owner:NITTO DENKO CORP
Methods of improving efficacy of allergy vaccines
InactiveUS20200188511A1Simple methodSsRNA viruses negative-senseAllergen ingredientsVirosomeSpecific immunity
Owner:ANERGIS +1
Adjuvant for desensitization vaccine and novel desensitization vaccine
ActiveCN108096575AAdjuvant effectAchieve enhanced effectOrganic active ingredientsAllergen ingredientsAdjuvantImmune tolerance
The invention provides an adjuvant for a desensitization vaccine; the adjuvant is prepared from components with immune tolerance; the components with immune tolerance are anti-inflammatory compounds.The invention also provides the novel desensitization vaccine using the anti-inflammatory compounds as the adjuvant. The anti-inflammatory compounds are used as the adjuvant and are co-injected with an allergen, so that inflammation of allergy vaccines is prevented at the injection site; the anti-inflammatory compounds have an effect of inhibiting the maturation of antigen-presenting cells; the anti-inflammatory compounds directly act on the antigen-presenting cells so as to inhibit the antigen-presenting cells from becoming mature cells during antigen uptake and presentation; the anti-inflammatory compounds realizes long-acting treatment of allergies by rapidly inducing the production of Treg cells. The desensitization vaccine provided by the invention can realize rapid, long-acting and effective treatment on the allergies from the view of sensitization mechanism.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
An adjuvant for desensitization vaccine and a new type of desensitization vaccine
ActiveCN108096575BRelieve symptomsDiffusion fastOrganic active ingredientsAllergen ingredientsAdjuvantMature cell
The invention provides an adjuvant for a desensitization vaccine, which includes an immune tolerance-inducing component, and the immune tolerance-inducing component is an anti-inflammatory compound. The present invention also provides a novel desensitizing vaccine using an anti-inflammatory compound as an adjuvant. In the present invention, the anti-inflammatory compound is used as an adjuvant and co-injected with the allergen to prevent the inflammation of the allergic vaccine at the site; the anti-inflammatory compound has the effect of inhibiting the maturation of antigen-presenting cells, and the anti-inflammatory compound directly acts on the antigen-presenting cells, inhibiting They become mature cells during antigen uptake and presentation; anti-inflammatory compounds achieve long-lasting treatment of allergies by rapidly inducing the generation of Treg cells. The desensitization vaccine provided by the invention can realize rapid, long-acting and effective treatment of allergic symptoms from the mechanism of sensitization.
Owner:CHENGDU MAXVAX BIOTECHNOLOGY LLC
Peptide carrier fusion proteins as allergy vaccines
The present invention relates to polypeptides comprising at least 3 peptide fragments consisting of 10 to 50 contiguous amino acid residues of at least 1 wild-type allergen fused to the Hepadnaviridae family N- and C-termini of viral surface polypeptides or at least 1 fragment fused to said surface polypeptides.
Owner:WORG PHARM (HANDZHOU) CO LTD
Allergy vaccines
InactiveUS20090191268A1Reduce IgE antibody effectHigh anti-self responsePowder deliveryCell receptors/surface-antigens/surface-determinantsAdjuvantAllergy
Owner:HELLMAN LARS T +2
Use of an adjuvanted allergy vaccine formulation for parenteral administration
The present invention relates to the use of a seasonal allergen composition for the manufacture of a vaccine formulation for preventing or treating allergy to the allergen composition in a subject byparenteral administration, wherein the vaccine formulation is administered in a dosage regimen comprising an up-dosing phase, wherein the up-dosing phase partly or wholly overlaps with the allergen season of the allergen composition.
Owner:ALK ABELLO SA
Pharmaceutical product for up-dosing of allergy vaccine
This invention relates to the use of a pharmaceutical product comprising allergen and optionally an adjuvant for fast up-dosing in connection with allergy vaccination wherein a reduced number of injections are used. The invention also relates to the pharmaceutical product as such.
Owner:ALK ABELLO SA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com