An adjuvant for desensitization vaccine and a new type of desensitization vaccine
A vaccine and desensitization technology, applied to allergic diseases, medical preparations containing active ingredients, antibody medical ingredients, etc., can solve problems such as inability to induce immune tolerance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
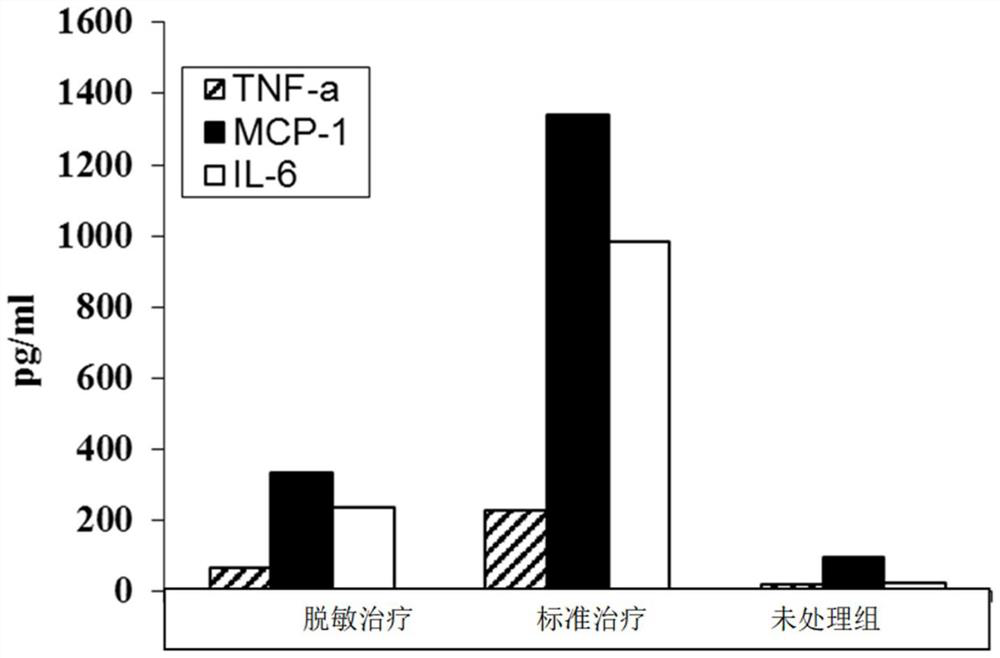
[0050]The desensitization vaccine formulation in Example 1 is a glucocorticoid (acetic acid dexamethasone) and egg white protein, and the vaccine is applied to the skin, and the inflammatory cytokine production of skin cells can be suppressed. Such asfigure 1 As shown, the level of inflammatory cytokines produced by the skin of the application of the drug. In Example 1, the application of the desensitization treatment group contained the egg white protein and glucocorticoid; the application of the standard treatment group only contains only the compound white protein; the apparest for the unprocessed group was physiological saline.
[0051]Example 1 The test uses 6 to 8 weeks large female BALB / C mice. Abdominal skin hair of mice shaved, rest for 24 hours. The desensitization treatment group mice were applied to hair removal skin; the second day, the liquid of the egg white protein was applied to the same part of the skin surface - the skin can be complete Or sandpaper treatment of ep...
Embodiment 2
[0056]Example 2 The dust mite allergy and desensitization treatment model of mice were established.
[0057]This test uses a 6 to 8 weeks of female C57BL / 6 mice (Charles River), or Balles River. The allergic model of the mouse involves two steps of sensitization and attack. The step of sensitization is to inject 50 μg of dust mites (adsorbed on the surface of 1 mg of aluminum hydroxide) in mice. The amount of injection is 0.5 ml. At 14 days, attack. The step of attacking the drug is to enter the lungs 10 μg HDM (HDM is dust mite extract) in the mouse driver. 48 hours in repeated attack once. After 24 hours of the second attack, carbon dioxide was used to eat mice. Bronchial alveolar lavewater (BALF) was collected, and the counting of various types of cells and cytokine analysis were collected, and the lung tissue was collected.
[0058]After the death of mice, the pulmonary was washed 3 times with 1 ml of sterile brine. The pulmonary washed liquid, and the supernatant was stored at -80 ...
Embodiment 3
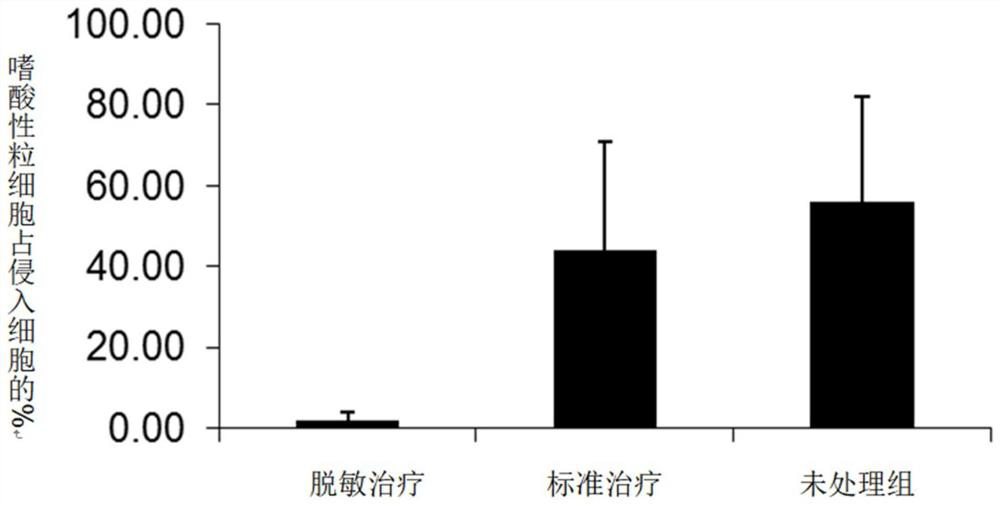
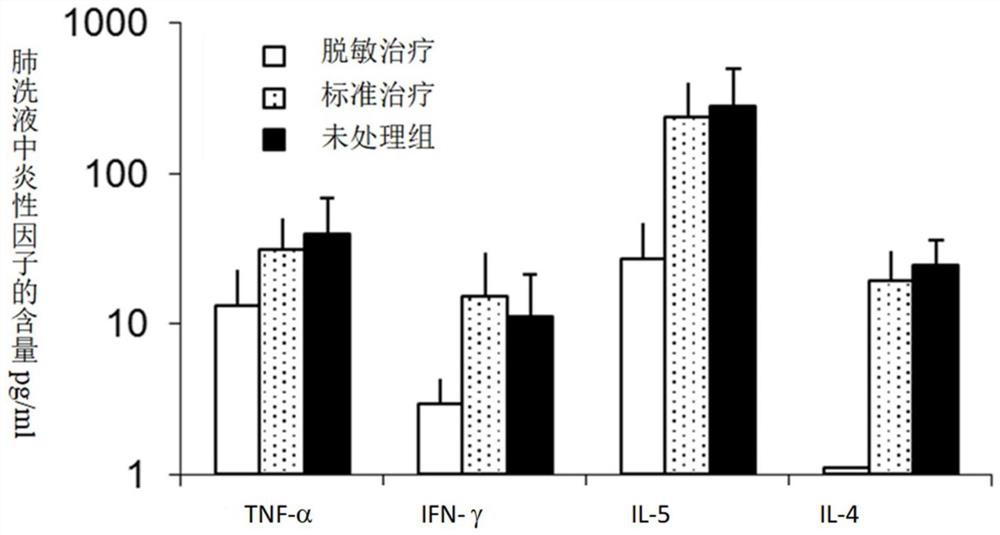
[0061]Example 3 utilizes a desensitization vaccine containing a glucocorticoid and an indoor dust mites, an allergic mouse obtained by immunotherapy is effective, reducing dust mites induced by immunotherapy. Such asfigure 2 Due to the mouse model of dust mites, desensitization treatment affects eosinophils in lung intracellularophilic in the lungs. The application of the desensitization treatment group is a dust mite extract, and the application of the glucocorticoid, the application of the standard treatment group contains only dust mites extract, and the application of the unreamped group is physiological saline. By using a desensitization vaccine containing glucocorticoid and indoor dust mites, mouse respiratory inflammation is reduced, especially the number of eosinophils in the lung, especially the number of eosinophils in the lung.image 3 Due to the mouse model of dust mites, desensitization therapy on the influence of pulmonary cytokines. Dental treatment group of allergens ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com