Peptide carrier fusion proteins as allergy vaccines
A carrier and vaccine technology, applied in the direction of carrier-antigen complex structure, vaccine, carrier-bound antigen/hapten components, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
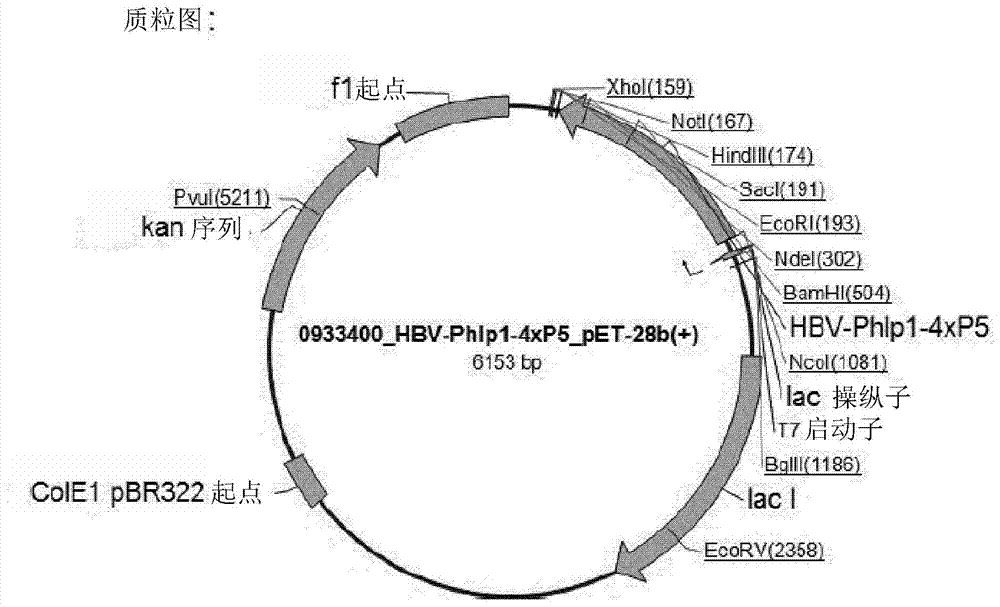
[0448] Embodiment 1: Construction of HBV Phlpl_4xP5 (BM321) expression plasmid
[0449] Synthetic BM321 genes were assembled from synthetic oligonucleotides and / or PCR products and cloned into an appropriate standard vector (pMK-RQkanR). Plasmids were purified from transformed E. coli K12 strain (DH10B-T1R), and concentrations were determined by UV spectroscopy. The final synthesized and codon-optimized BM321 DNA sequence was further cloned into the expression vector pET28b(+) using appropriate restriction sites (NcoI site at the 5' end and EcoRI at the 3' end). Plasmid DNA was purified from transformed E. coli K12DH10B (dam+dcm+), and the concentration was determined by UV spectroscopy. The final construct was confirmed by sequencing of the insert. A summary of the plasmid data and plasmid map of the final expression vector "pBM-321" is shown below.
[0450] Summary of the BM321 sequence cloned into the final expression vector pET-28b(+).
[0451] sequence
Embodiment 2
[0452] Embodiment 2: the transformation of expression plasmid to the expression host that is used for HBV_Phlpl_4xP5 (BM321)
[0453] Chemically competent E. coli BL21(DE3) cells were transformed with expression plasmids by heat shock method. Transformed cells were plated on LB agar plates consisting of 0.5% sodium chloride, 1% soy peptone, 0.5% yeast extract, 1.5% agar and 50 μg / mL kanamycin for selection. Cells were grown on LB plates by overnight incubation at 37°C. Individual clones of transformed BL21(DE3) E. coli cells were isolated, cultured in LB medium, and screened for growth and expression of BM321. The best performing clones were selected for further master cell bank establishment.
Embodiment 3
[0454] Embodiment 3: Preparation of the master cell bank of HBV Phlpl_4xP5 (BM321)
[0455] Aliquots of selected clones were used for inoculation of 150 mL of medium (composition: 0.5% NaCl, 1% soy peptone, 0.5% yeast extract, 50 μg / mL kanamycin). Incubate the master cell bank (MCB) culture at 37 °C with continuous agitation at 200 rpm until the culture reaches the optical density OD 600 =1-2. Glycerol was added to obtain a final glycerol concentration of 15% v / v, the MCBs were aliquoted into 1 mL vials and stored in a -75 ± 10 °C ultra-low temperature freezer.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com