Allergy vaccine composition, production method thereof and use of same in allergy treatment
A vaccine composition and allergen technology, applied in the field of immunology, to achieve the effect of reducing the number of injections and shortening the time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1. Acquisition and purification of mite allergens
[0052] Allergens were derived from whole mite cultures, including dead mites, scales, faecal particles, and low molecular weight media components. Extract the allergen in one of the following solutions: ammonium bicarbonate, sodium bicarbonate, phosphate-buffered saline, or saline with a pH near physiological. Crude extracts were clarified by centrifugation and filtration, and low molecular weight components were removed by gel filtration chromatography (Sephadex G-25) or diafiltration (cut-off: 5-10 kD) or both. Subsequently, the product was concentrated by ultrafiltration and freeze-dried to ensure its stability. For further purification of the allergen, the freeze-dried extract was resuspended in aqueous solution and subjected to salting out precipitation in 50-100% ammonium sulfate solution. The precipitate was resuspended in water and separated by gel filtration chromatography on Superdex 75. Intermedia...
Embodiment 2
[0053] Embodiment 2. Obtaining of proteoliposome
[0054] It starts with a N. meningitidis B culture. Biomass was harvested by centrifugation and extracted using detergents, enzymes and sonication. Cellular debris was removed by centrifugation and the supernatant was treated with nuclease digestion to remove nucleic acids. The extract was recovered by ultracentrifugation, resuspended in detergent solution, and purified by molecular separation chromatography to remove the remaining low and intermediate molecular weight components. The proteoliposomes obtained contained less than 10% nucleic acid and about 10% LPS embedded in their structure; but no free form. Optionally, capsular polysaccharide is added in a ratio of 0.5 to 4 times the proteoliposome content, which is derived from Gold and Gotschlich (Gold et al., 1969-1970, WHO Bull.45:279-282 and Gotschlich et al., 1969, J . Exp. Med. 129: 1349-1365) of Neisseria meningitidis serogroup C. Optionally, aluminum hydroxide is...
Embodiment 3
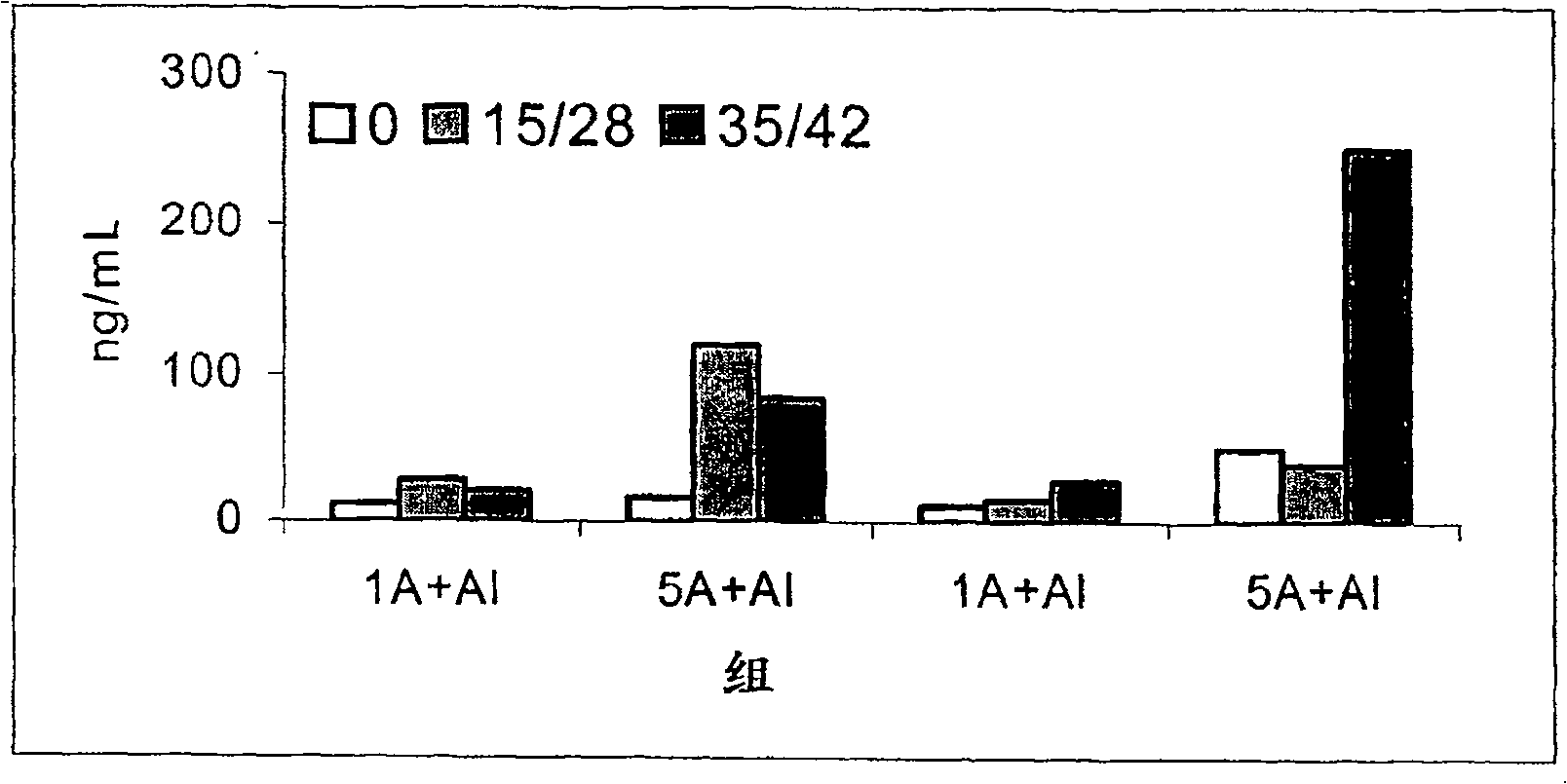
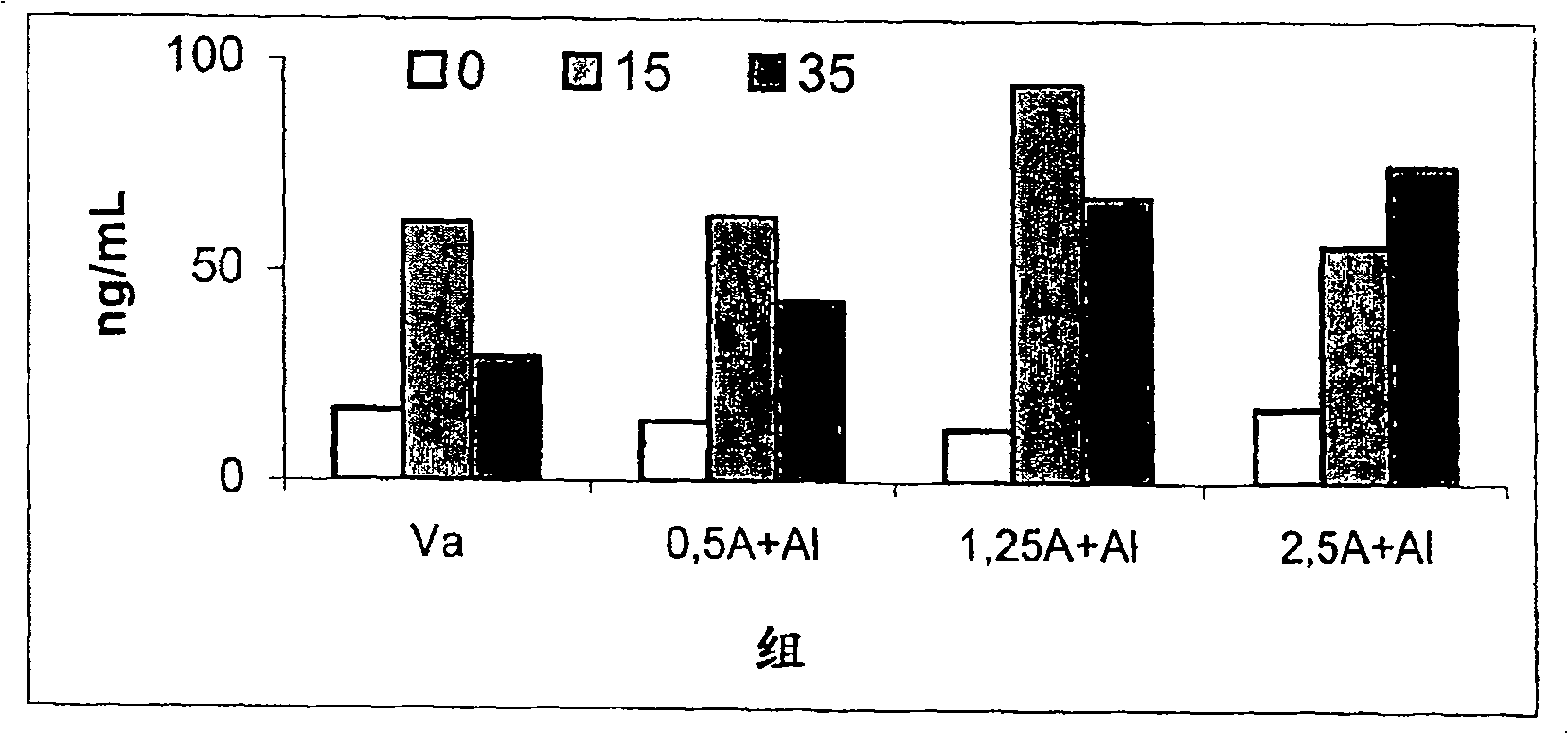
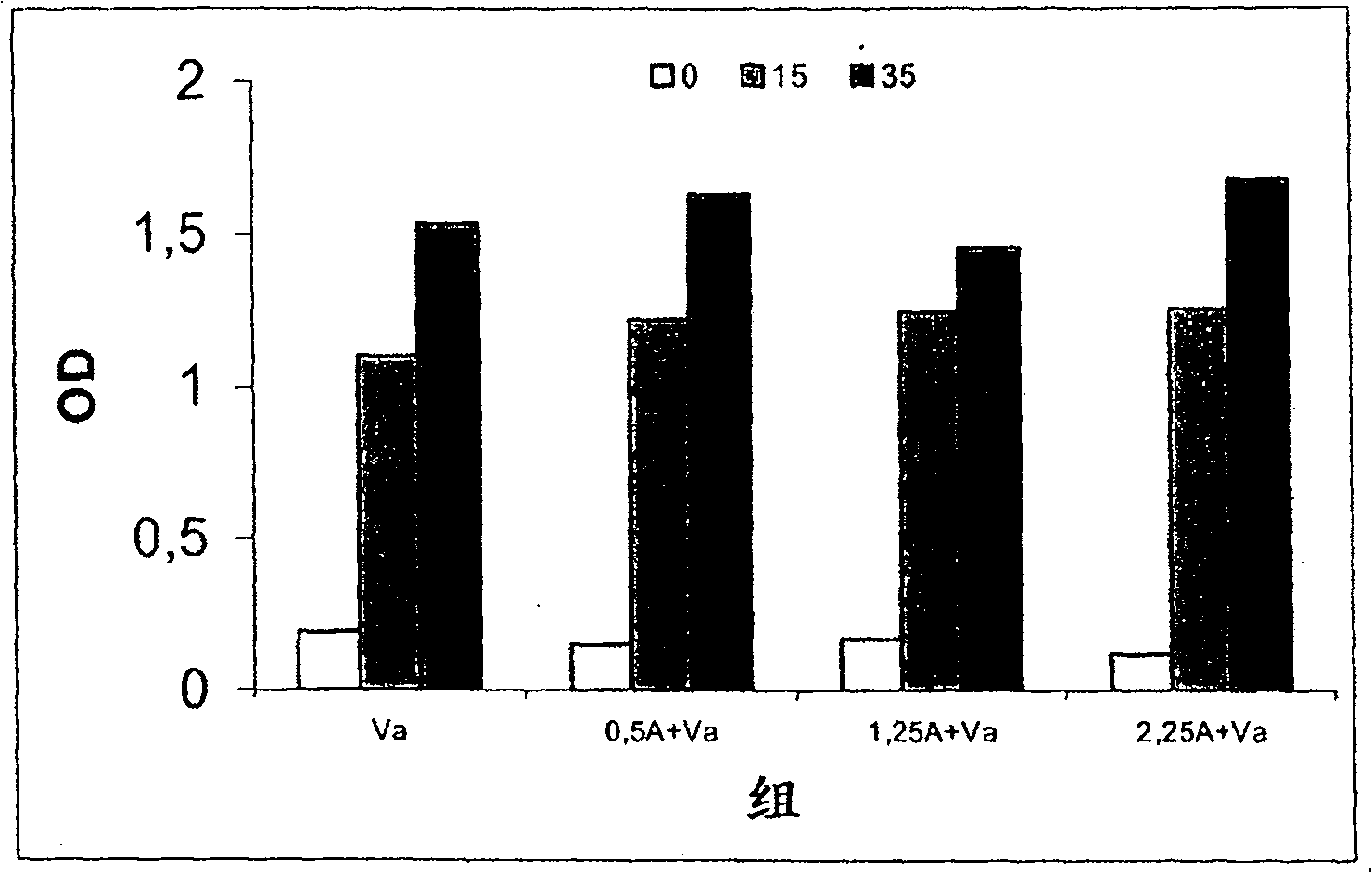
[0055] Example 3. Determination of Allergenicity (IgE Binding Activity) of Preparations Adsorbed on Aluminum Hydroxide
[0056]Allergenicity, expressed as the binding capacity of human IgE antibodies, can be determined by IgE-inhibition ELISA. This assay measures the ability of a sample solution to inhibit the binding of IgE to a solid phase coated with an allergen. In this case, the sample preparation used was D. siboney allergen purified according to Example 1, mixed with proteoliposomes obtained according to Example 2, and then adsorbed onto aluminum hydroxide gel. The initial Der s 1 allergen concentration was 4 μg / mL and the allergen activity was 400 BU / mL (BU: biological unit defined according to the 2nd edition of the Nordic Guidelines for the Registration of Allergenic Products. 10000 BU / mL in the SkinPrick Test Equivalent to 10mg / mL histamine hydrochloride.)
[0057] ELISA was performed according to the procedure described below. Polystyrene microplates were coated...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com