A kind of acyclovir hydrocortisone cream and preparation method thereof
A technology for hydrocortisone and pine cream, which is applied in the directions of medical preparations containing active ingredients, ointment delivery, pharmaceutical formulations, etc. Process and other problems, to achieve the effect of strong operability of the preparation process, shortened production cycle, and good process repeatability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A kind of acyclovir hydrocortisone cream, its raw material formula is as follows:
[0045] Acyclovir 5.0%
[0046] Hydrocortisone 1.0wt%
[0047] Poloxamer 188 1wt%
[0048] Citric acid 0.75wt%
[0049] Sodium hydroxide 0.25wt%
[0050] Cetearyl alcohol 12wt%
[0051] White Vaseline 7.5wt%
[0052] Liquid paraffin 7.5wt%
[0053] Sodium lauryl sulfate 1wt%
[0054] Propylene glycol 20wt%
[0055] Purified water balance
[0056] The steps of the method for preparing acyclovir hydrocortisone cream from the raw materials of the above formula are as follows:
[0057] (1) Weigh the oil phase components cetearyl alcohol, white petrolatum and liquid paraffin respectively according to the raw material formula, and heat to 75-80°C and mix to obtain the oil phase.
[0058] (2) Dissolve sodium hydroxide, poloxamer 188 and sodium lauryl sulfate in purified water, heat to 75-80°C, and then add acyclovir to dissolve to obtain an aqueous phase.
[0059] (3) Add the water phase to the oil phase, stir and ...
Embodiment 2
[0064] A kind of acyclovir hydrocortisone cream, its raw material formula is as follows:
[0065] Acyclovir 5.0%
[0066] Hydrocortisone 1.0wt%
[0067] Poloxamer 188 0.5wt%
[0068] Citric acid 0.3wt%
[0069] Sodium hydroxide 0.1wt%
[0070] Cetearyl alcohol 8wt%
[0071] White Vaseline 5wt%
[0072] Liquid paraffin 5wt%
[0073] Sodium lauryl sulfate 0.5wt%
[0074] Propylene glycol 10wt%
[0075] Purified water balance
[0076] The preparation method is the same as in Example 1.
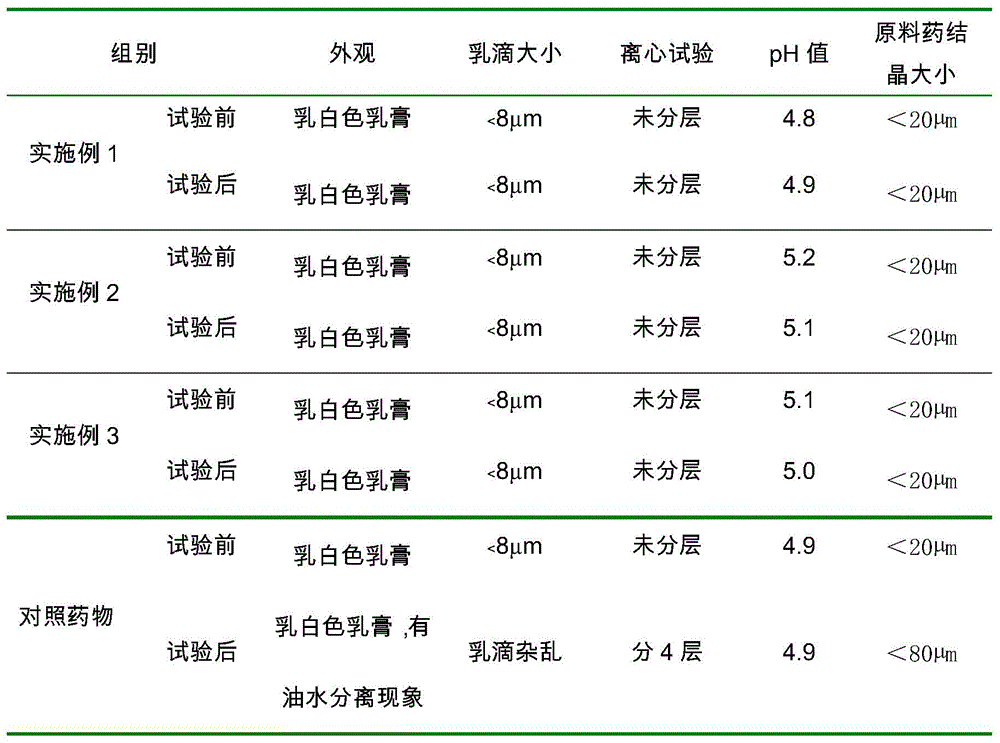
[0077] The prepared cream is milky white, pH 5.2, microscopic observation, the bulk drug crystals are less than 20 μm, evenly dispersed in the cream matrix; the particle size of the emulsion droplets are all less than 8 μm, centrifuged at 3000 rpm for 30 minutes, without layering or breaking.
Embodiment 3
[0079] A kind of acyclovir hydrocortisone cream, its raw material formula is as follows:
[0080] Acyclovir 5.0wt%
[0081] Hydrocortisone 1.0wt%
[0082] Poloxamer 188 2wt%
[0083] Citrate 3wt%
[0084] Sodium hydroxide 1wt%
[0085] Cetearyl alcohol 16wt%
[0086] White Vaseline 10wt%
[0087] Liquid paraffin 10wt%
[0088] Sodium lauryl sulfate 2wt%
[0089] Propylene glycol 10wt%
[0090] Purified water balance
[0091] The preparation method is the same as in Example 1.
[0092] The prepared cream is milky white, pH 5.1, microscopic observation, the bulk drug crystals are less than 20 μm, evenly dispersed in the cream matrix; the particle size of the emulsion droplets are all less than 8 μm, centrifuged at 3000 rpm for 30 minutes, without layering or breaking.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com