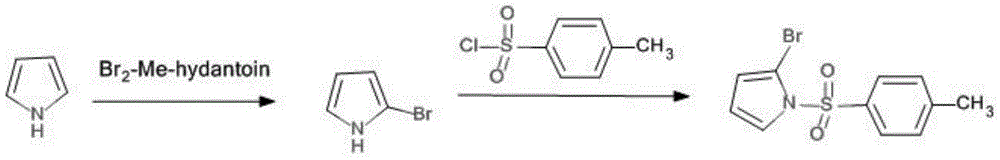
A method for synthesizing 2-bromo-n-p-methylbenzenesulfonylpyrrole
A technology of toluenesulfonylpyrrole and toluenesulfonyl chloride, which is applied in the field of synthesizing 2-bromo-N-p-methylbenzenesulfonylpyrrole, can solve the problem of 2-bromopyrrole with a purity of only 85% and cannot be separated and purified. Instability and other problems, to achieve the effect of easy reaction, easy industrial production, and good purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] One, the preparation of N-p-toluenesulfonate pyrrole:
[0022] Add 100g, 1.491mol of pyrrole and 165.96g, 1.64mol of triethylamine into a 3L reaction flask, then add 1500mL of anhydrous ether, cool to 0°C, slowly add 284.10g, 1.491mol of p-toluenesulfonyl chloride dropwise, and react The system was warmed up to room temperature, reacted at room temperature for 6 h, added saturated NH4Cl solution, separated the organic phase, washed with water, washed with saturated brine, dried with anhydrous magnesium sulfate, and distilled off the solvent to obtain a white solid compound;
[0023] Two, the synthesis of 2-bromo-N-p-toluenesulfonate pyrrole:
[0024] Under the protection of nitrogen, add 100g, 0.45mol N-p-toluenesulfonate pyrrole and 1000mL tetrahydrofuran to a 3L reaction flask, cool to -78°C, slowly add 292mL, 0.50mol, 1.7Ninhexanes tert-butyl lithium dropwise, and complete the addition , reacted at 78°C for 3h, then added dropwise 47.88g, 0.45molBrCN in 100mL tetrah...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com