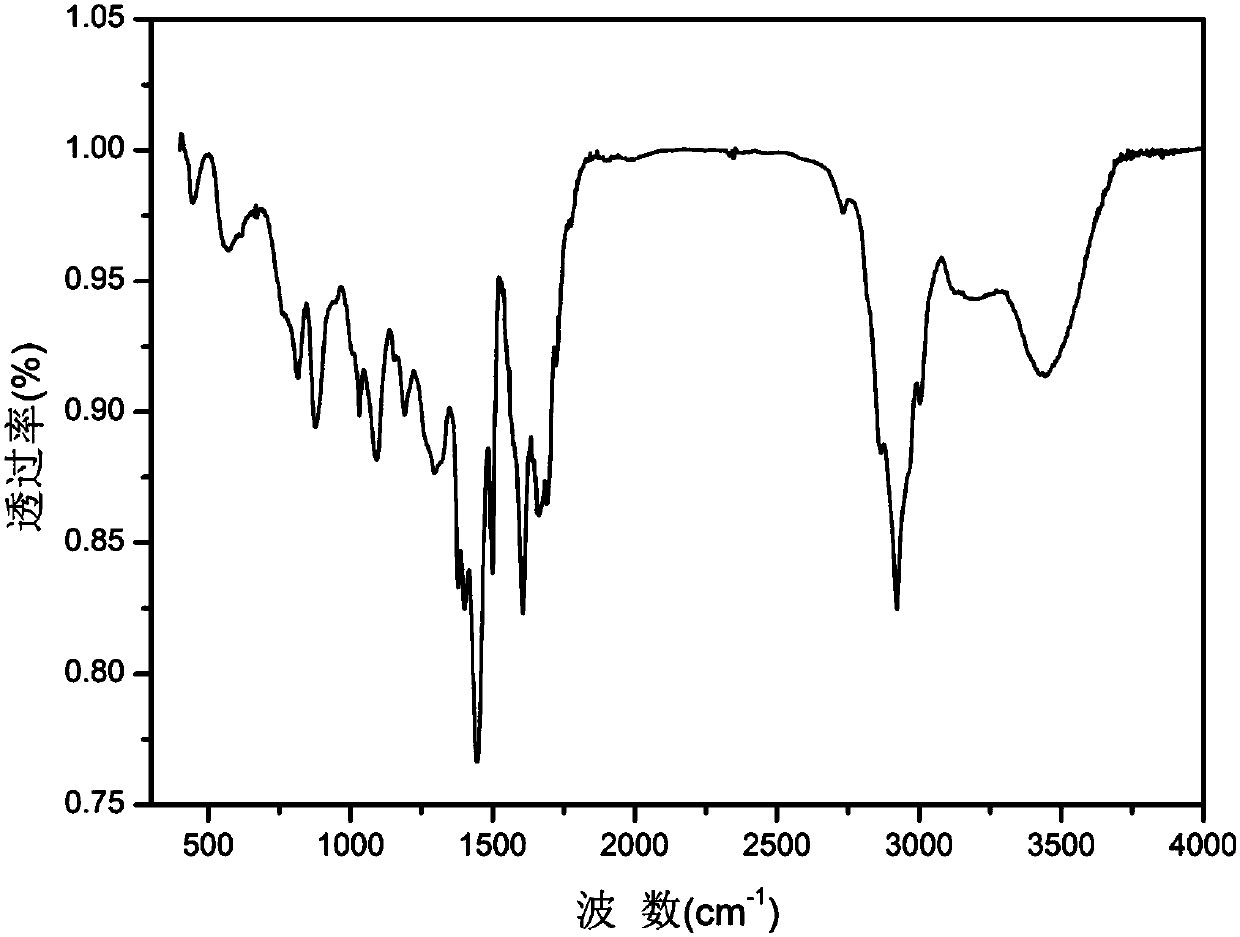
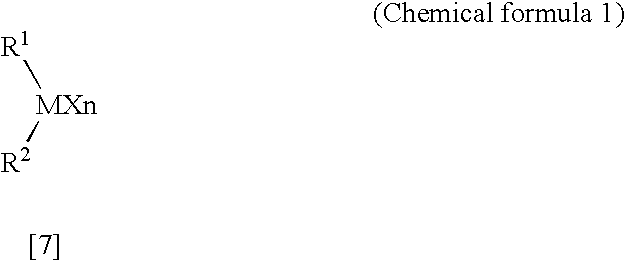
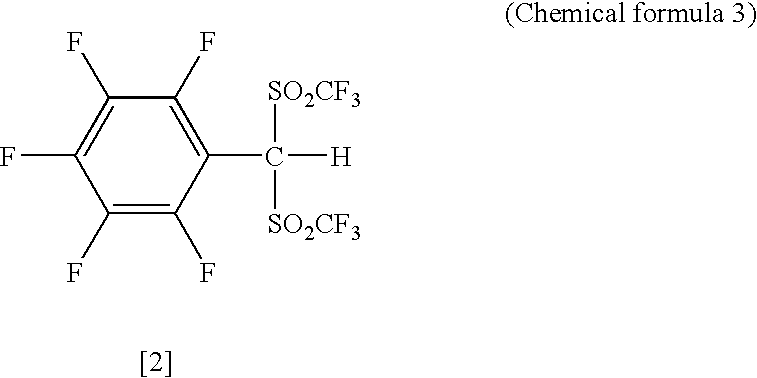
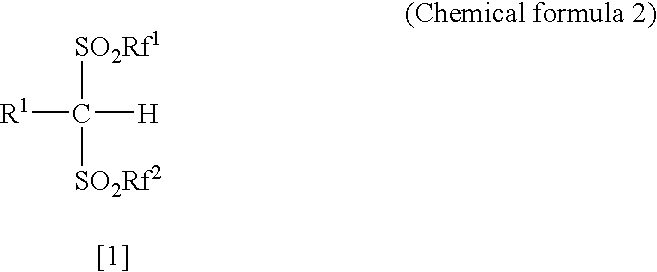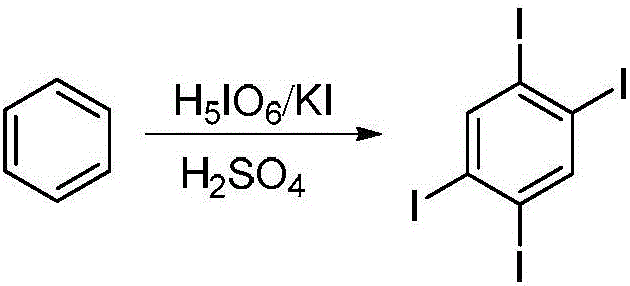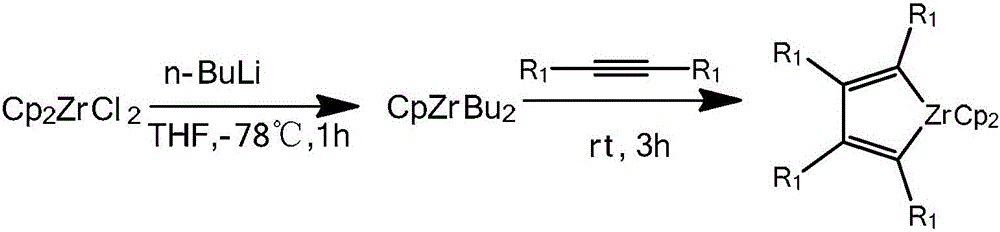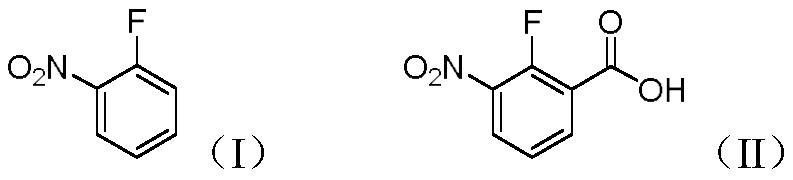Patents
Literature
34 results about "Tert-Butyllithium" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tert-Butyllithium is a chemical compound with the formula (CH₃)₃CLi. As an organolithium compound, it has applications in organic synthesis since it is a strong base, capable of deprotonating many carbon acids, including benzene. tert-Butyllithium is available commercially as hydrocarbon solutions; it is not usually prepared in the laboratory. Its synthesis was first reported by R. B. Woodward in 1941.
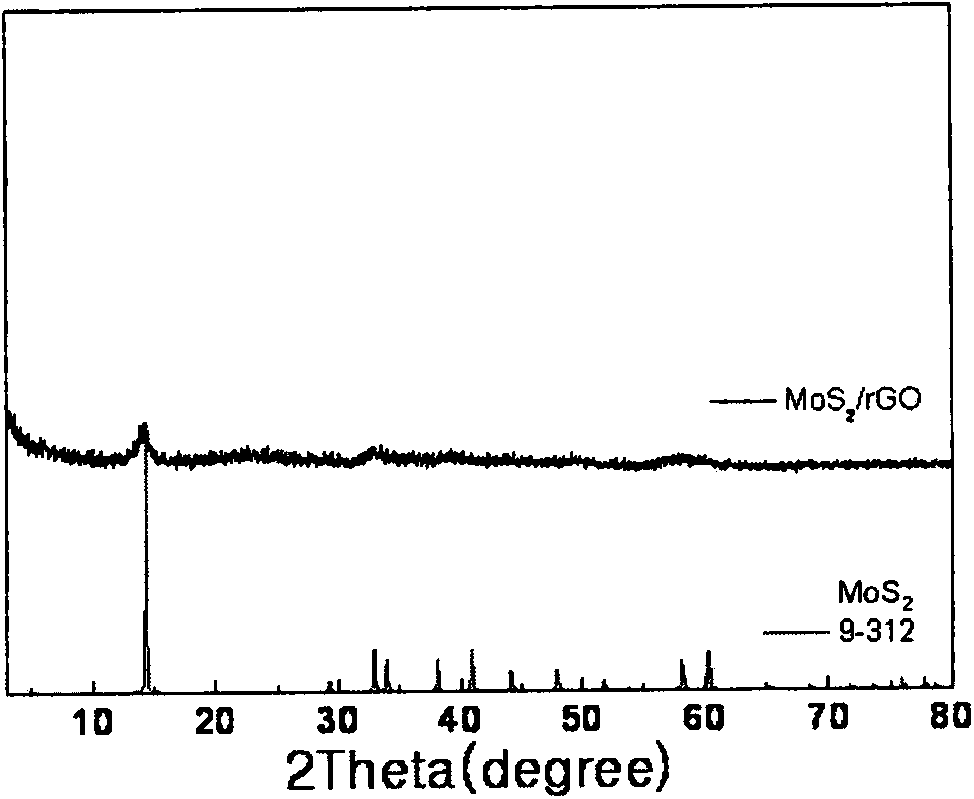
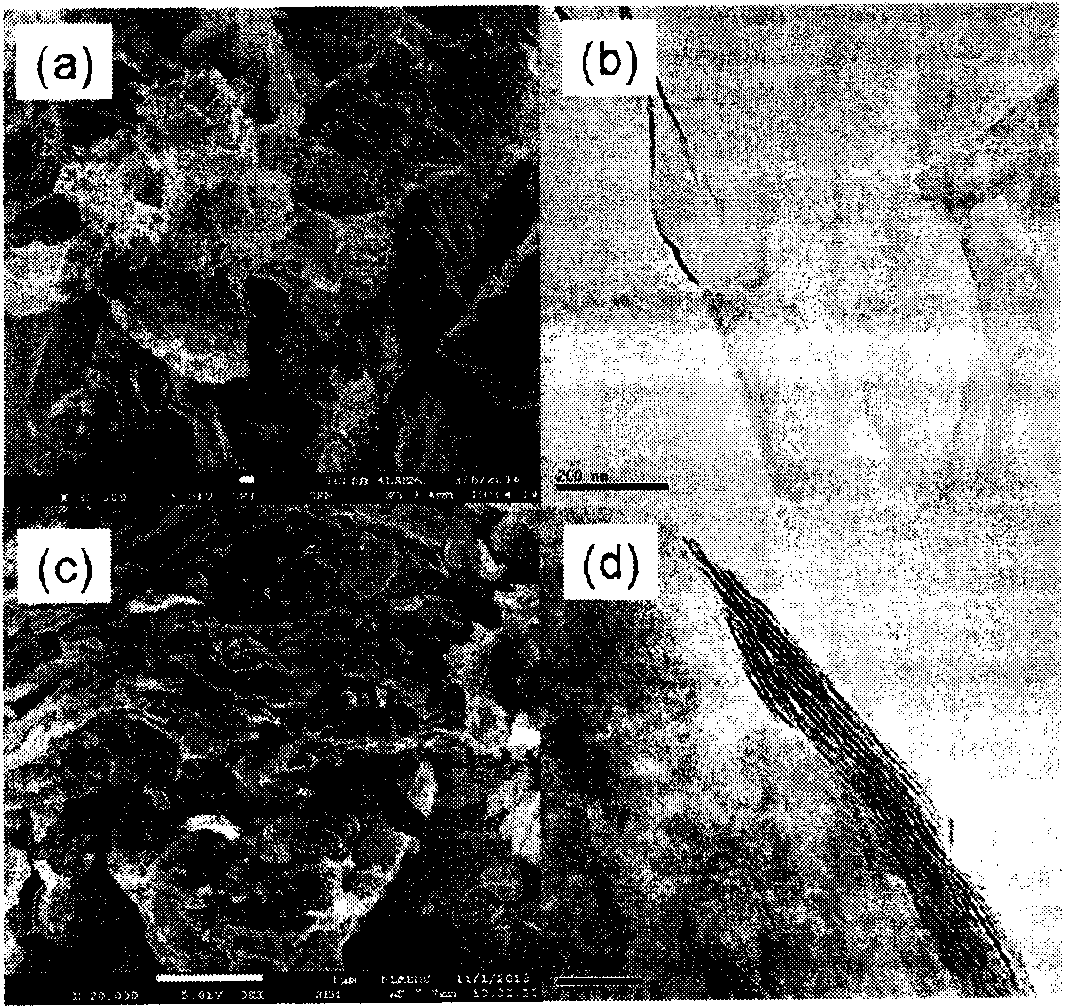
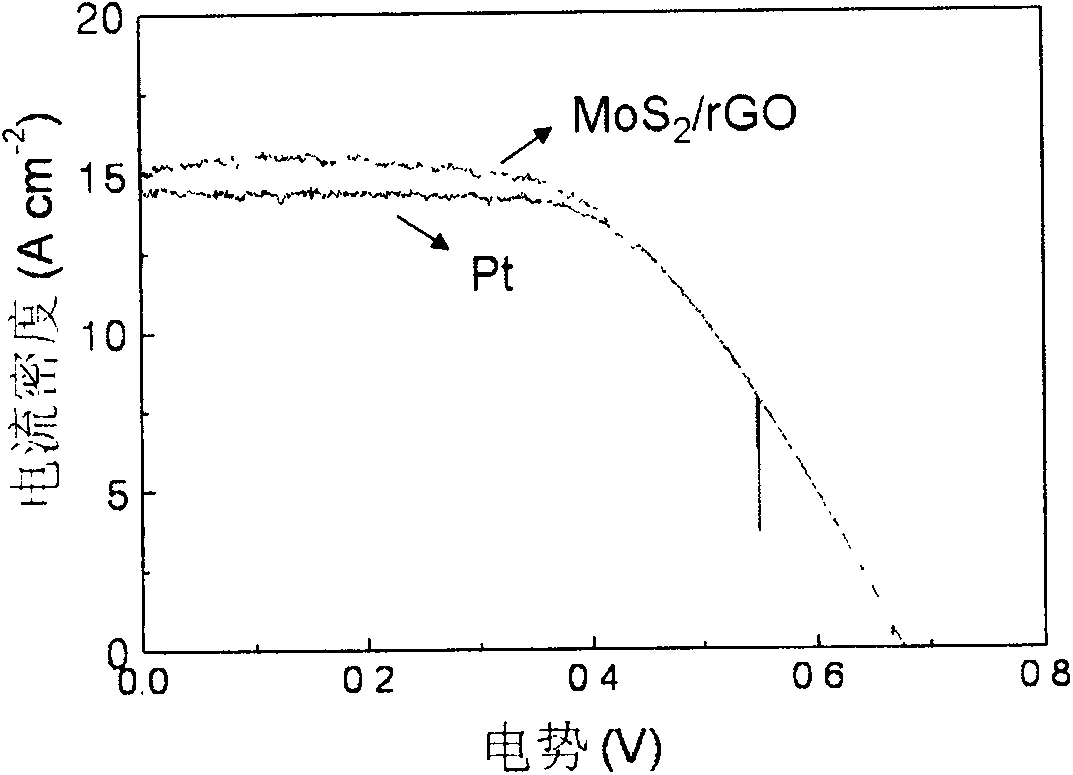
Counter electrode made of metal sulfide and graphene composite materials and preparation method and application of counter electrode
ActiveCN103903861AImprove conductivityImprove catalytic performanceLight-sensitive devicesFinal product manufactureRoom temperatureTert-Butyllithium
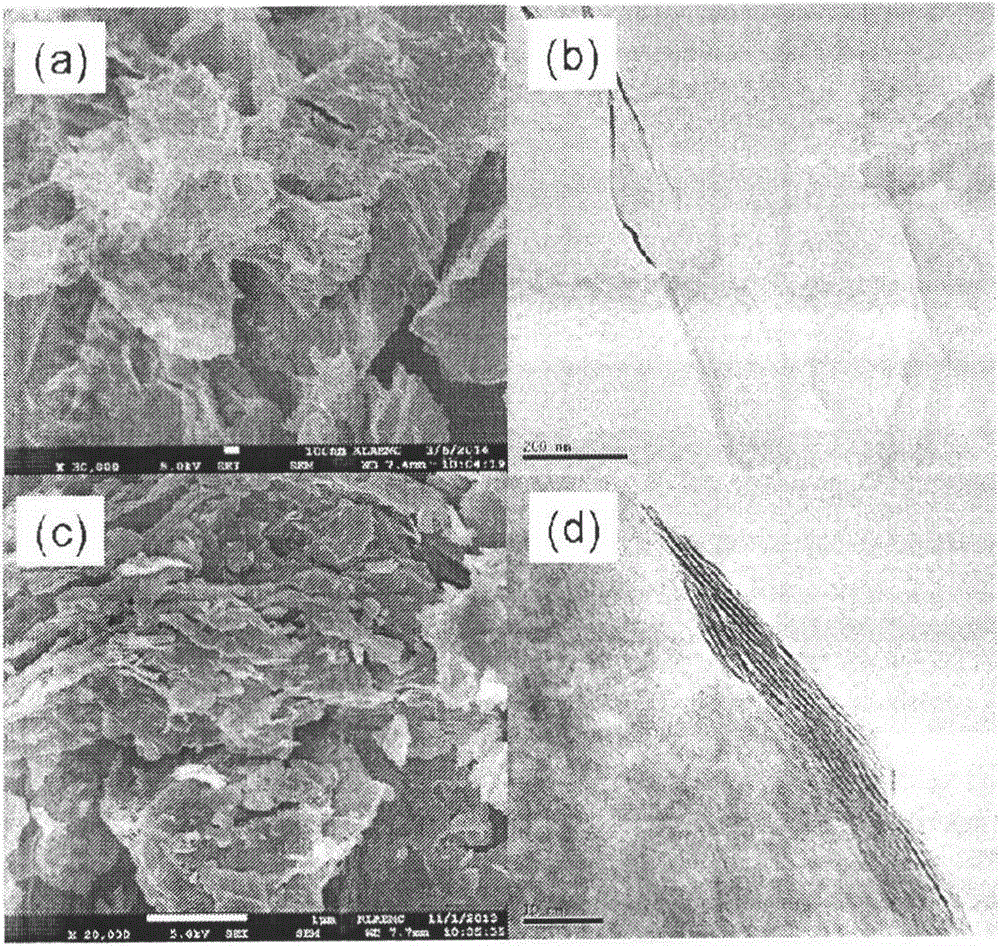
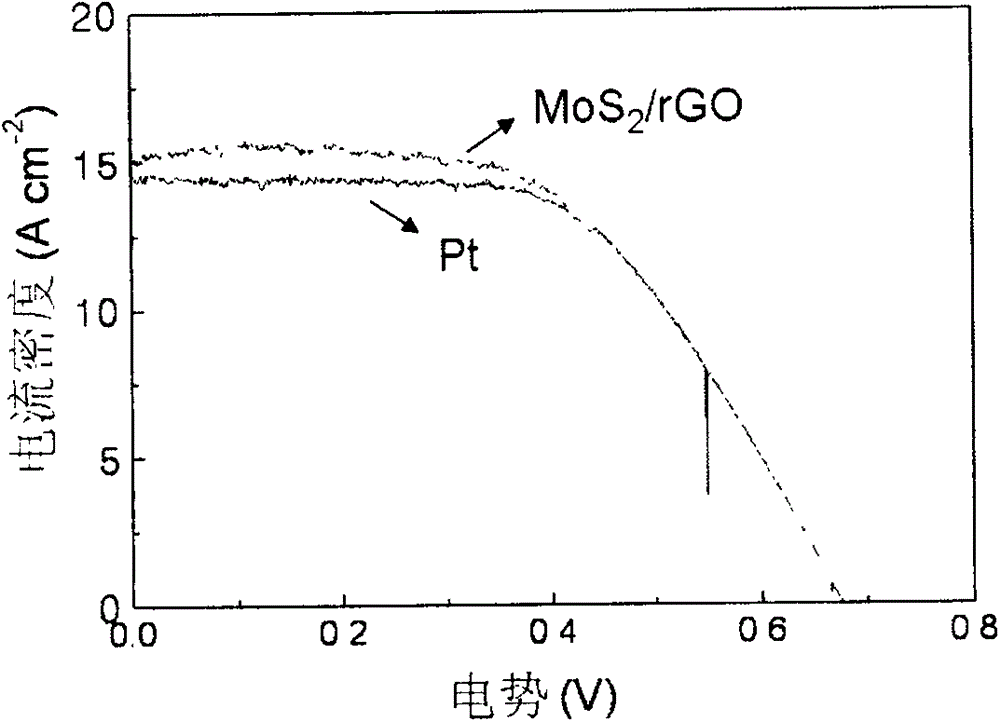
The invention relates to a counter electrode made of two-dimensional metal sulfide and graphene composite materials, a preparation method of the counter electrode and application of the counter electrode in dye sensitization solar cells. Metal sulfide and graphene composites are loaded on a conductive substrate for preparation. The metal sulfide and graphene composites are dissolved in a solution and are deposited to form a film after being filtered, compressed film plating is carried out on the conductive substrate, drying is carried out, and then the obtained counter electrode is cooled to be at a room temperature, wherein the mass ratio of the metal sulfide to graphene is 20:1 to 20, and the mass ratio of sulfide to tert-butyllithium is 1:5 to 50. Binding agents do not need to be added, and accordingly high-temperature impurity removing is not needed, and the appearance and the structure of the materials can be well maintained. Compared with the material of a Pt counter electrode, the materials are richly reserved in nature, and industrial production can be carried out on a large scale. Compared with materials of other counter electrodes, the materials are easy and convenient to prepare and excellent in catalytic performance, and accordingly the two-dimensional metal sulfide and graphene composite materials have wide application prospects in the field of the dye sensitization solar cells.
Owner:NANKAI UNIV
Catalyst for preparing n-Butyl butyrate by n-butanal and preparation method thereof
ActiveCN107649180AImprove stabilityReusableOrganic-compounds/hydrides/coordination-complexes catalystsPreparation by aldehyde oxidation-reductionPolymer scienceN-Butyllithium
The invention relates to a catalyst for preparing n-Butyl butyrate by n-butanal and a preparation method thereof. The catalyst is prepared by the following steps: using a hydroxy-enriched porous organic polymer as a substrate, and forming a complexation structure of Ru on the surface of the substrate; firstly preparing a hydroxy-enriched porous organic polymer material POP; adding tert-butyl lithium or n-butyl lithium, and preparing a polymer POP-Li; and adding ruthenium salt to obtain a polymer POP-Ru. The catalyst has the characteristics of good stability, repeated use, and higher reaction conversion rate and selectivity. The preparation method is capable of creatively fixing Ru ions on the surface of the catalyst, a new idea is provided for the preparation of the catalyst, and the catalyst has the significant application value.
Owner:TIANJIN BOHUA YONGLI CHEM IND
Arylbis (perfluoroalkylsulfonyl)methane and metallic salt thereof, and methods for producing the same
InactiveUS20050070741A1High yieldEasy to getOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsTrifluoromethanesulfonic anhydrideLithium
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis (trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
Metal phase molybdenum disulfide, electrode, preparation method, electrocatalyst and energy storage element
InactiveCN111115686APromote formationSuitable for mass productionPhysical/chemical process catalystsNegative electrodesPropanoic acidPtru catalyst
The invention relates to the field of materials, and particularly discloses metal-phase molybdenum disulfide, an electrode, a preparation method, an electrocatalyst and an energy storage element. Themetal-phase molybdenum disulfide comprises the following raw materials: a molybdenum source, a sulfur source, an organic acid and a proper amount of water; wherein the organic acid is selected from acetic acid or propionic acid. According to the metal-phase molybdenum disulfide provided by the embodiment of the invention, the organic acid is used as an additive to promote the formation of the metal-phase molybdenum disulfide; the content of the metal-phase molybdenum disulfide in the product can reach 72%. The method has the advantages of being simple, safe and suitable for large-scale production, and the problems that existing synthesis of the metal-phase molybdenum disulfide mostly adopts tert-butyl lithium as a raw material, equipment is complex, safety is low, and the production periodis long are solved. The preparation method provided by the embodiment of the invention is low in required reaction temperature, short in reaction time, simple in equipment, energy-saving, safe in used medicines and suitable for large-scale production.
Owner:JILIN UNIV
Preparation method and application of intermediate for preparing fluocalcitol
ActiveCN111960938AReduce usageRaw materials are cheap and easy to getGroup 4/14 element organic compoundsOrganic compound preparationPhosphonium saltSodium amalgam
The invention discloses a preparation method and application of an intermediate for preparing fluocalcitol, and belongs to the field of organic chemistry. The preparation method comprises the following steps: by taking a compound 1 as an initial raw material, carrying out addition reaction, tertiary hydroxyl protection, ester group reduction and iodination on the compound 1 and a compound 2 to obtain a compound 6, reacting the compound 6 with triphenylphosphine to form a quaternary phosphonium salt, carrying out Wittg reaction on the quaternary phosphonium salt and a compound 8 to obtain an olefin compound 9, reducing, removing a silyl ether protection group and oxidizing to obtain an intermediate 12; the raw materials are cheap and easy to obtain, the reaction steps are simplified, the preparation cost is reduced, the yield is high, and the product quality is easy to control; use of dangerous samples such as tert-butyl lithium, sodium amalgam, tri-n-butyl hydrogen stannate and carbondisulfide is avoided, the preparation risk is reduced, and large-scale preparation is easy.
Owner:甘肃皓天医药科技有限责任公司
Efficient high-stereoselectivity synthesis process of polysubstituted 3-cyclopenten-1-one
InactiveCN1348950AHigh yieldEfficient and high yieldPreparation by carbon monoxide reactionCyclopenteneKetone
This invention is a high-effective high-steroselectivity synthetic method for multi-substituted 3-cyclopentene-1-ketone. It is incldues the following steps: firstly, the derivative of 1,4-diiodo-1,3-butadiene dissolved in the solvent of ethyl ether or tetrachlorofuran reacts with n-butyl lithium or tert-butyl lithium under low temp., CO is further introduced,l quenching reaction is conducted, andthe pure product is obtained further through extracting, stripping, drying, concentrating and decontaminating. The said invented method is scientific and rational, is a most direct and most simple method, and the yield is high, and the product is easily decontaminated.
Owner:席振峰 +2
(R)-tert-butyl dimethyl siloxy-glutaric acid monoester preparation method
ActiveCN104370953AChiral naturalGood chiralityGroup 4/14 element organic compoundsHydroxybutyric acidGlutaric acid
The invention discloses a rosuvastatin intermediate (R)-tert butyl dimethyl siloxy-glutaric acid monoester preparation method, which belongs to the field of medicine. The method is as follows: compound (S)-4-halogen-3-hydroxy butyric acid ethyl ester is taken as a raw material for ester exchange to obtain other (S)-4-halogen-3-hydroxy butyric ester, (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate is obtained by protection of the (S)-4-halogen-3-hydroxy butyric ester, and by condensation of the (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate and chloroformate in the presence of magnesium, tert butyl lithium and n-butyl lithium, (R)-tert butyl dimethyl siloxy-glutaric acid ester benzyl ester is obtained, and the (R)-tert butyl dimethyl siloxy-glutaric acid monoester is obtained by catalytic hydrogenation deprotection. Compared with the original process, the raw material of the process is chiral, natural, needs no resolution and induction and no enzyme hydrolysis to produce the chiral center, so that the product chirality is better. Compared with the original process, enzymes are already-industrialized enzymes, the raw material is easily obtained, reaction conditions are relatively mild, and the process is easy to industrialize. Compared with the original process, the process is shorter and less in three wastes.
Owner:ZHEJIANG LEPU PHARMA CO LTD
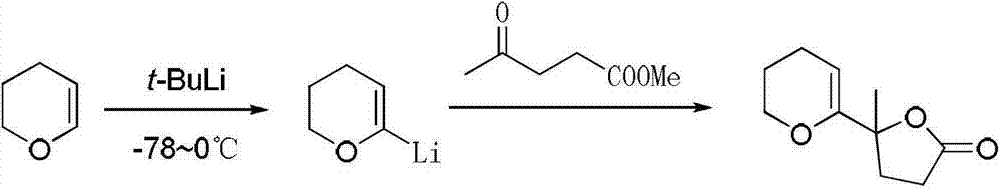
Preparation method of tert-butyllithium solution
The invention discloses a preparation method of a tert-butyllithium solution, which comprises the following steps: (1) in an inert gas protective atmosphere, adding sodium-lithium alloy into an inert solvent, heating while stirring to disperse the sodium-lithium alloy, cooling, and removing the inert solvent to obtain a sodium-lithium alloy dispersion; and (2) in an inert gas protective atmosphere, adding pentane into the sodium-lithium alloy dispersion obtained in the step (1), dropwisely adding a chlorinated n-butane-chlorinated tert-butyl alkane mixed solution at 30-38 DEG C while stirring to carry out reaction, and after the reaction is complete, treating to obtain the tert-butyllithium solution. By adding the catalytic amount of chlorinated n-butane into chlorinated tert-butyl alkane, the reaction for preparing the tert-butyllithium can be initiated and carried out smoothly, thereby implementing industrialized-scale production of the tert-butyllithium.
Owner:SHANGYU HUALUN CHEM
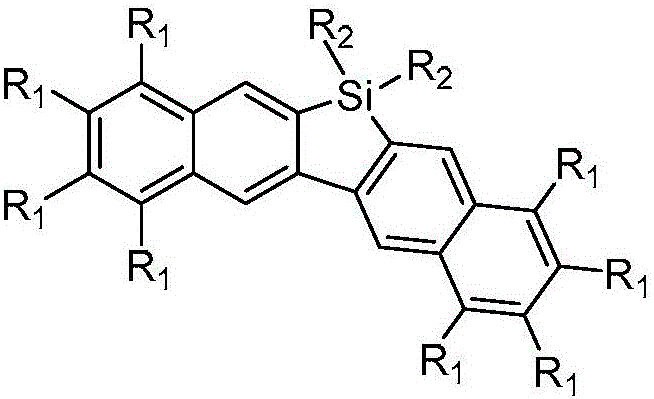
Dinaphthosilole organic photoelectric functional material and synthesis method thereof
InactiveCN106543215ASimple methodHigh stereoselectivitySilicon organic compoundsLuminescent compositionsSynthesis methodsActivation action
The invention discloses a dinaphthosilole organic photoelectric functional material. The functional material is synthesized mainly through the following steps: synthesizing a zirconium heterocycle performing water-free and oxygen-free reaction on bis-substituted internal alkyne under the catalytic action of a metallocene zirconium complex; under the catalytic action of cuprous chloride, reacting with tetraiodo-benzene to generate diiodo-naphthalene; synthesizing a dinaphthalene under the lithiation action of n-butyllithium; and finally synthesizing the dinaphthosilole photoelectric material under the lithiation action of tert-butyllithium. In the synthesis process, the method for preparing the diiodo-naphthalene through coupling reaction of heterocyclopentadiene and polyarylated iodide is simple and high in yield; through the activation action of the n-butyllithium, the dinaphthalene can be synthesized from the bimolecular diiodo-naphthalene; and the metallocene zirconium complex is used as a homogeneous olefin polymerization catalyst, so that the catalytic activity and the olefin polymerization stereoselectivity are high. The synthesized dinaphthosilole is of great use in the fields of conductive high-polymer materials, liquid crystal materials and biological active substances.
Owner:TIANJIN UNIV
Arylbis (perfluoroalkylsulfonyl) methane, metal salf of same, and processes for producing these
InactiveCN1481357AOrganic compound preparationOrganic-compounds/hydrides/coordination-complexes catalystsArylTrifluoromethanesulfonic anhydride
The present invention provides a method for producing various types of arylbis(perfluoroalkylsulfonyl)methane having a bulky aryl group and an electron-accepting aryl group in which synthesis was conventionally considered to be difficult, at high efficiency; a novel arylbis(perfluoroalkylsulfonyl)methane that can be widely applied to asymmertric catalyst, various types of functional materials and the like; and a metallic salt thereof. In addition, excellent catalysts are also provided. An aryl halomethane is reacted with a sodium trifluoromethane sulfinate, the arylmethyl triflone produced thereby is reacted with a t-BuLi and the like, the lithium salt of the arylmethyl triflone obtained is reacted with a trifluoromethane sulfonic acid anhydride, and an arylbis(trifluoromethylsulfony)methane such as pentafluorophenylbis(triflyl)methane, {4-(pentafluorophenyl)-2,3,5,6-tetrafluorophenyl}bis(triflyl)methane and the like are obtained at a high yield.
Owner:JAPAN SCI & TECH CORP
Reaction system for safely adding tert-butyllithium into reactor
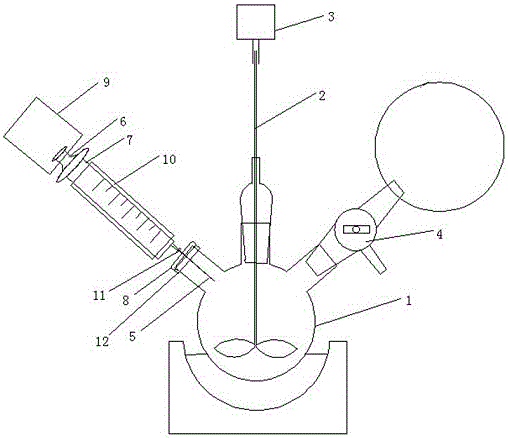
InactiveCN106422985ASimple structureReasonable designChemical/physical/physico-chemical stationary reactorsInert gas productionBiochemical engineeringTert-Butyllithium
The invention provides a reaction system for safely adding tert-butyllithium into a reactor. The reaction system comprises a reaction device, a cooling device, a gas protection device and an injector. The reaction device comprises a reaction bottle, a stirring paddle tightly connected to an opening in the middle of the reaction bottle in a sleeved mode and a motor arranged at the upper end of the stirring paddle; the cooling device is arranged under the reaction bottle; the gas protection device is connected with an opening in the right side of the reaction bottle through a three-way valve; the injector comprises a syringe needle, a piston and a needle cylinder, the syringe needle is inserted into a rubber plug to be connected with an opening in the left side of the reaction bottle, the piston is connected with an air cylinder, a cooling layer is arranged on the periphery of the needle cylinder, a reinforcing device is arranged on the periphery of the joint of the syringe needle and the needle cylinder, and a fastening device is arranged on the periphery of the joint of the rubber plug and the opening in the left side of the reaction bottle. The reaction system has the advantages of being simple in structure, reasonable in design, high in automation degree, capable of saving time and labor, safe and convenient in sampling, good in sealing effect and wide in application and has the wide application range.
Owner:南京睿宇物联网科技有限公司
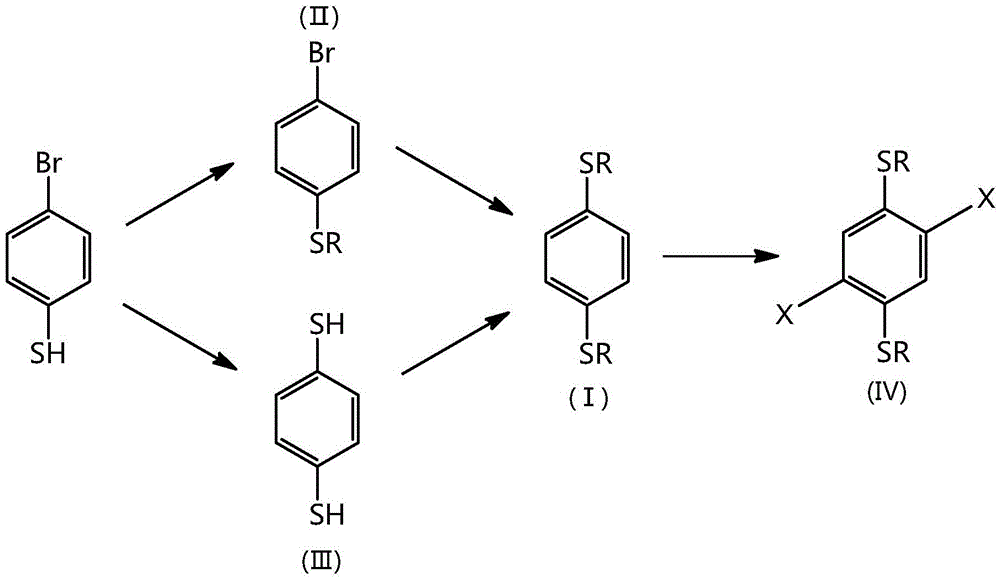
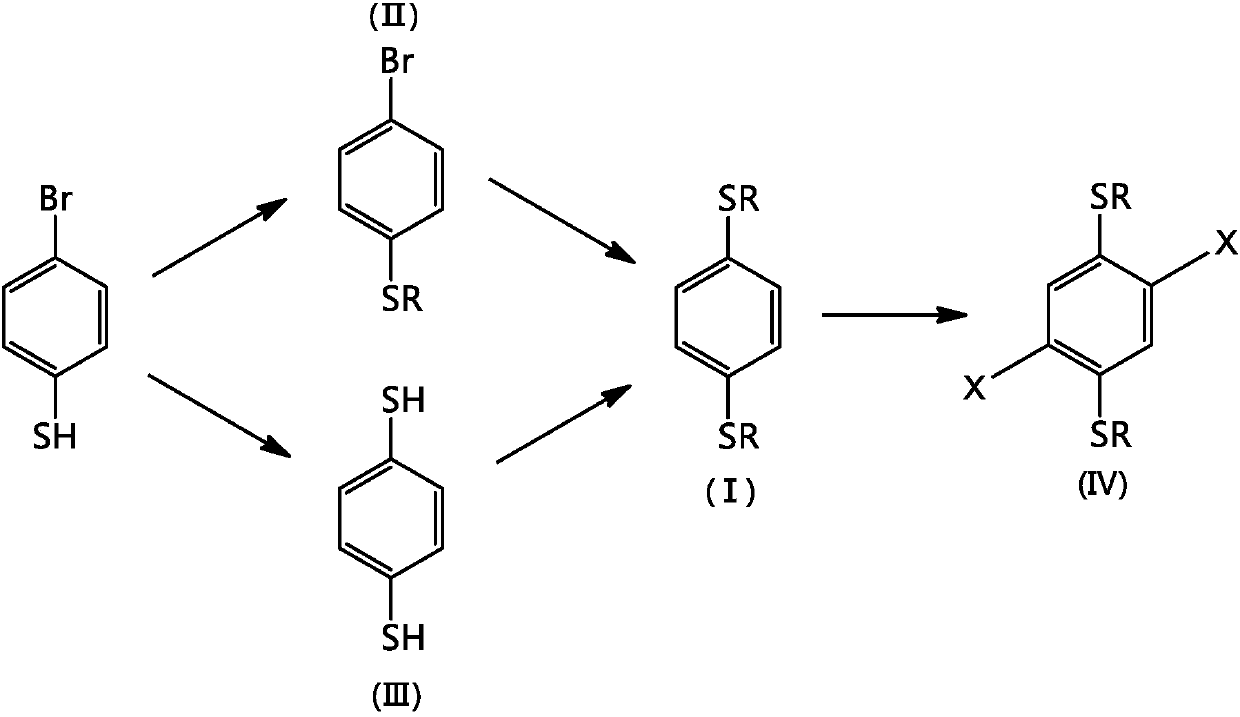
Synthetic method for 1,4-dithioalkyl benzene and halide thereof
InactiveCN106380431AImprove securityImprove maneuverabilityThiol preparationHydropoly/poly sulfide preparationAlkaneBenzene
The invention relates to a synthetic method for 1,4-dithioalkyl benzene and halide thereof. According to the method, 4-bromothiophenol is used as a raw material and reacts with alkyl bromide to synthesize 4-bromo-1-thioalkyl benzene, and then a reaction is carried out to synthesize 1,4-dithioalkyl benzene; or 4-bromothiophenol successively reacts with n-butyl lithium, sulfur powder and acid to synthesize 1,4-benzenedithiol, and then1,4-benzenedithiol reacts with alkyl bromide to synthesize 1,4-dithioalkyl benzene; and 1,4-dithioalkyl benzene can be further halogenated. The synthetic method provided by the invention does not need flammable and combustible t-butyl lithium, so the synthetic method has better security and controllability, is favorable for large-scale industrial production, reduces production cost and is environment friendly.
Owner:XIANGTAN UNIV
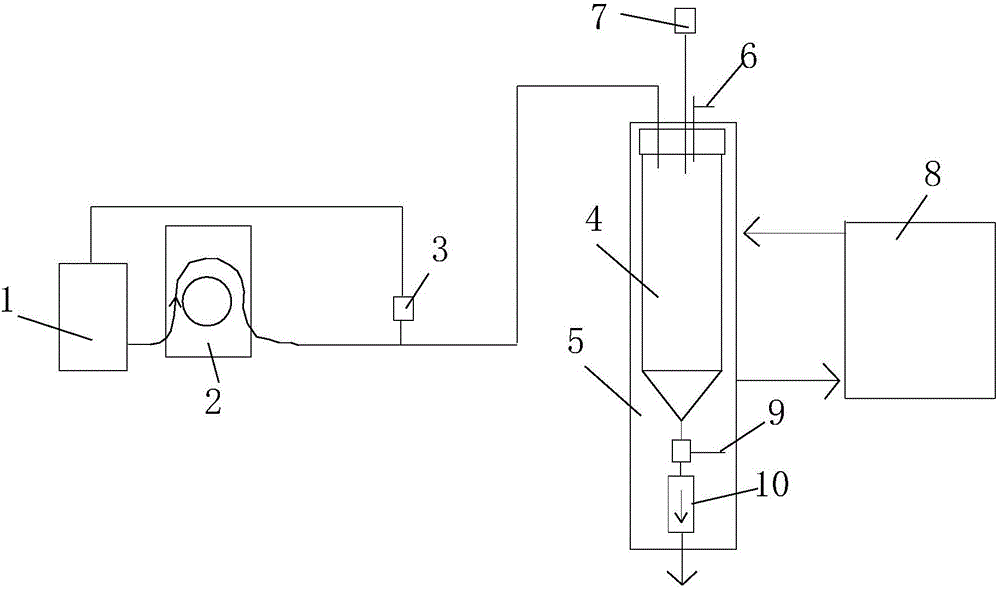
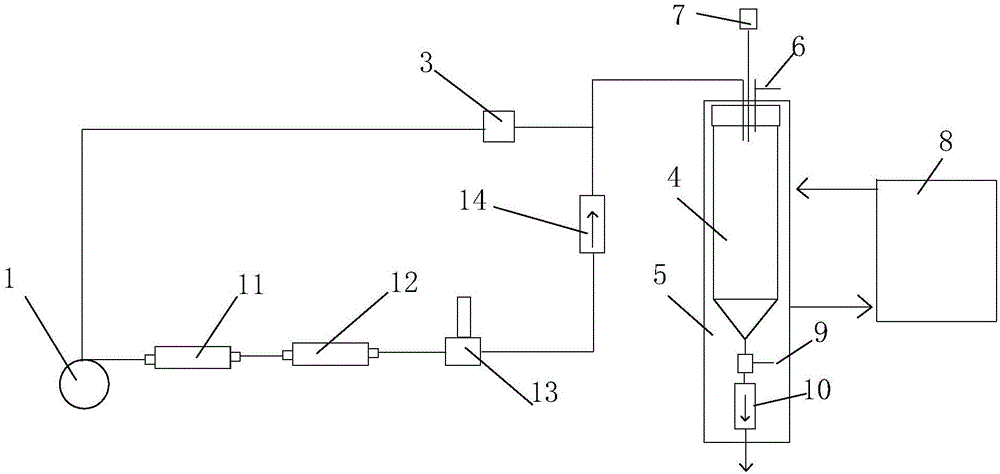
Dangerous liquid metering system and metering and conveying method
The invention discloses a dangerous liquid metering system and a metering and conveying method. The dangerous liquid metering system comprises a metering container, a protection fluid storage tank and a fluid metering and conveying device, wherein the top part of the metering container is provided with a protection fluid inlet and an overflow outlet; an overflow valve is arranged at the overflow outlet; the fluid metering and conveying device is used for metering and conveying dangerous liquid or protection fluid; the bottom part of the metering container is provided with a dangerous liquid outlet, and the exterior of the metering container is provided with a heating system for heating the metering container. The dangerous liquid metering system has the advantages that the dangerous liquid can be metered and conveyed; under the condition of avoiding the dangerous liquid touching air, the high-accuracy metering and conveying can be realized; the system can be widely applied to the fields of analysis and synthesis of yellow phosphor, tert-butyllithium, and other highly flammable matters, and the like.
Owner:苏州阿洛斯环境发生器有限公司
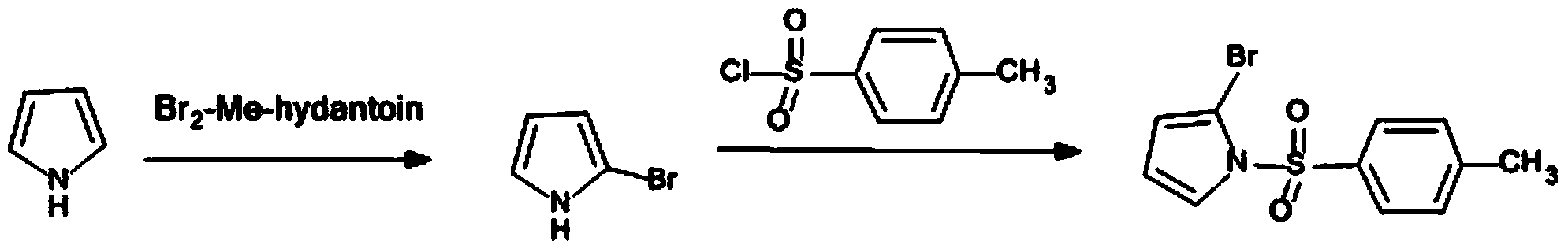
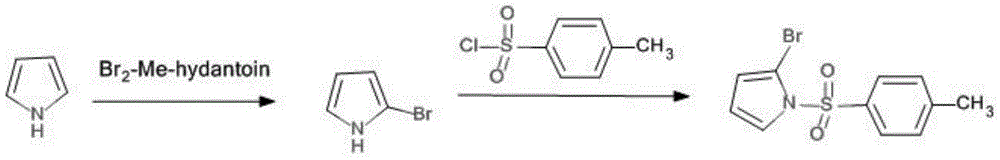
Method for synthetising 2-bromine-N-methyl benzenesulfonyl pyrrole
The invention discloses a method for synthetising 2-bromine-N-methyl benzenesulfonyl pyrrole, relates to organic synthetic chemistry, and belongs to the field of medicines and chemical engineering. The method is characterized by comprising the following steps: firstly, a benzenesulfonyl reaction is conducted on pyrrole nitrogen to generate a stable intermediate compound; then, tert-butyllithium is allowed to highly selectively be subjected to lithiation reaction with ortho-position hydrogen of N-methyl benzenesulfonyl pyrrole; finally, the product is allowed to react with BrCN to obtain the target compound. The method has the benefits that the yield of the target compound is high, the total yield of the two steps of reactions reaches more than 50%, the purity is high, the reactions are easy to operate, the performance is high, and the industrial production is easy to realize.
Owner:成都安斯利生物医药有限公司
A method for synthesizing 2-bromo-n-p-methylbenzenesulfonylpyrrole
Owner:成都安斯利生物医药有限公司
1-(2,3,5,6-tetrafluorophenyl)-2,2-dimethylacetone compound and its preparation method
ActiveCN103864593BExtended service lifeReduce consumptionLithium organic compoundsCarbonyl compound preparation by hydrolysisLithiumN-Butyllithium
The invention provides a 1-(2, 3, 5, 6-tetrafluorophenyl)-2, 2-dimethyl acetone compound. The structural formula of the 1-(2, 3, 5, 6-tetrafluorophenyl)-2, 2-dimethyl acetone compound is shown in the specification. A synthetic method of the compound comprises the steps that 1, 2, 4, 5-phenyl tetrafluoride serving as a raw material exchanges with n-butyllithium so as to generate 2, 3, 5, 6-tetrafluorophenyl lithium, addition reaction happens between 2, 3, 5, 6-tetrafluorophenyl lithium and tert-butyl formonitrile, and finally the product is obtained through hydrolysis; another synthetic method of the compound comprises the steps that addition reaction happens between 2, 3, 5, 6-phenyl tetrafluoride formonitrile serving as a raw material and tert-butyllithium, and finally the target product is obtained through hydrolysis. The compound provided by the invention has the advantages of short synthetic time and low cost in an application process.
Owner:大连鼎燕医药化工有限公司
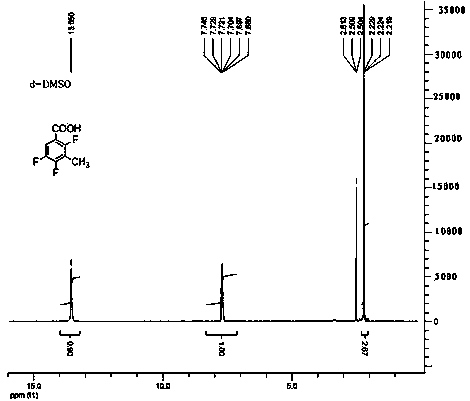
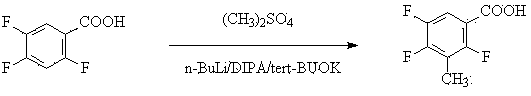
Method for preparing 2,4,5-trifluoro-3-methyl benzoic acid
ActiveCN102531887BReasonable designHigh yieldOrganic compound preparationCarboxylic compound preparationBenzoic acidEthylenediamine
Owner:锦州三溢科技有限公司
Method for preparing lithium phosphate film through atomic layer deposition
PendingCN111304631ASolve the problem of forming a good coating effectPrecise thickness controlChemical vapor deposition coatingChemical adsorptionPhysical chemistry
The invention discloses a method for preparing a lithium phosphate film through atomic layer deposition. The method is characterized in that the atomic layer deposition technology is adopted; carriergas flow rushes tert-butyllithium pulses into a vacuum reaction chamber to have chemical self-saturation adsorption with a base material to be plated and have an exchange reaction to generate a lithium displacement precursor on the surface of the base material to be plated; and the lithium displacement precursor has a reduction reaction with trimethyl phosphate to generate the single lithium phosphate film. As the precursor has self-saturability in chemical adsorption, deposition of one single lithium phosphate film is completed within one process cycle. Each time the process cycle is repeated, one lithium phosphate film monolayer is laminated on the previous single lithium phosphate film. By controlling the number of process cycles, the thickness of the lithium phosphate film is controlled accurately. The step coverage rate of the lithium phosphate film is good. The good covering effect can be formed on a sample of a complex space structure, and a plating layer is smooth and high in uniformity and stability. The defects in the prior art are overcome.
Owner:江苏迈纳德微纳技术有限公司
Method for detecting components of mixed gas in wastewater pool
PendingCN112834467AEasy to detectImprove permeabilityOrganic chemistryFluorescence/phosphorescenceQuinolineSilica gel
The invention discloses a method for detecting components of mixed gas in a wastewater pool, which comprises the following steps: firstly, mixing 6-methoxy-2-methylquinoline and iodomethane, and performing heating for reaction to obtain faint yellow solid powder; dissolving 7-diethylamino-2-oxo-2H-benzopyran-3-formaldehyde and the product of the faint yellow solid powder prepared in the step 1, stirring and refluxing the mixture, performing cooling to the room temperature, performing filtering, collecting filtrate, performing reduced-pressure spin-drying, and performing silica gel column chromatography separation to obtain an orange solid powder SO2 probe; adding 3, 7-didimethylamino-5, 5-dimethyl diphenyl and tetrahydrofuran, performing cooling, dropwise adding tert-butyl lithium, performing stirring, and performing staying overnight; adding an HCl solution into the mixed solution, performing treating, dissolving residues in trifluoroacetic acid, and performing stirring overnight; continuously alkalifying, extracting, drying and evaporating the obtained solution; and purifying the residue by column chromatography to obtain the dark blue solid NO probe. By adopting a fluorescence analysis technology, the content of the mixed gas in the wastewater pool is easily detected, the response time is short, the sensitivity is high, and the detection range is wide.
Owner:江苏安泰安全技术有限公司
A kind of preparation method of tert-butyllithium solution
Owner:SHANGYU HUALUN CHEM
Preparation method of 2, 5-disubstituted-1, 4-terephthalaldehyde
ActiveCN112538007AHigh reactivityImprove Quantization EfficiencyOrganic compound preparationCarbonyl compound preparation by oxidationHexamethylphosphoramideBiochemical engineering
The invention belongs to the technical field of organic synthesis, and particularly relates to a preparation method of 2, 5-disubstituted-1, 4-terephthalaldehyde The 2, 5-disubstituted-1, 4-terephthalaldehyde disclosed by the invention is prepared by carrying out oxidation reaction on 2, 5-disubstituted-1, 4-dibromobenzene and DMF under the catalytic action of hexamethylphosphoramide (HMPA) and tert-butyl lithium. According to the method, tert-butyl lithium is added into a reaction system, so that the reaction activity is greatly improved, the reaction yield is greatly improved and is 78% or above under the combined action of tert-butyl lithium and HMPA by virtue of a trace amount of HMPA and the existence form of stabilized carbocations, and the product quantification efficiency is greatly improved.
Owner:NINGBO POLYTECHNIC
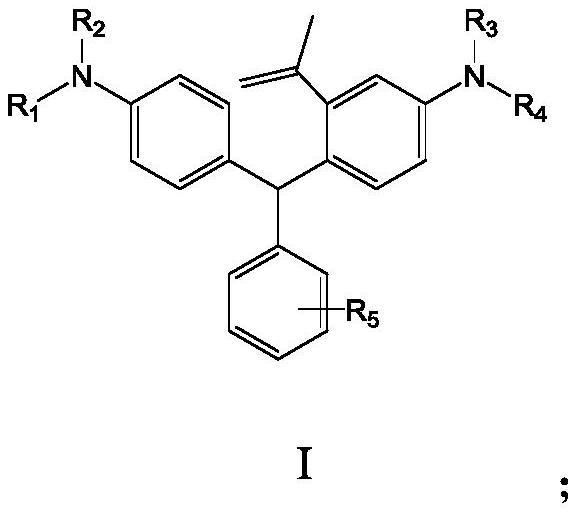
Benzene ring-containing compound as well as preparation method and application thereof
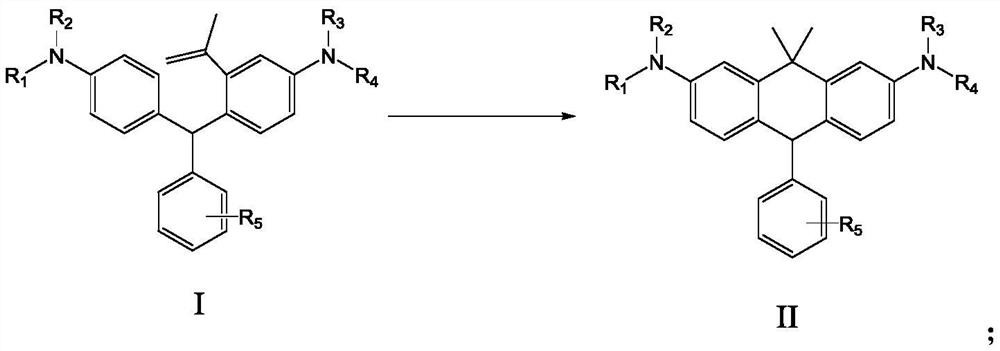
PendingCN114763323AMild reaction conditionsImprove securityAmino preparation from aminesOrganic compound preparationBenzeneAryl
The invention discloses a benzene ring-containing compound as well as a preparation method and application thereof. The structure of the benzene ring-containing compound provided by the invention is shown as a formula I; wherein R1, R2, R3 and R4 are independently a C1-6 alkyl group, a C3-6 cycloalkyl group or a C6-20 aryl group; and R5 is hydrogen, halogen, C1-C4 alkyl,-COOMe,-COOEt or carboxyl. According to the compound containing the benzene ring, the carbon rhodamine compound can be obtained under the mild condition, strong oxidants such as potassium permanganate and perchlorate do not need to be used, and the use and storage problems of dangerous chemicals such as tert-butyllithium are not involved.
Owner:SHENZHEN HUADA GENE INST
Preparation method of 2-fluoro-3-nitrobenzoic acid
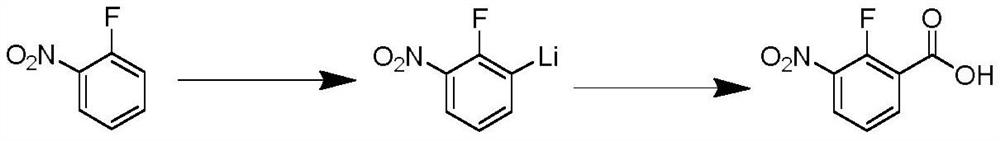
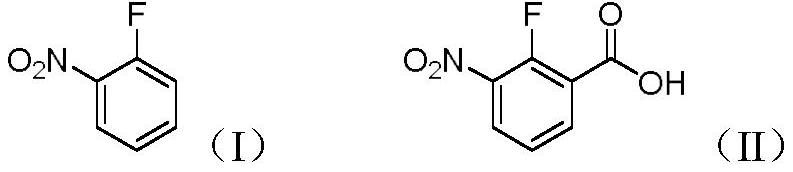
PendingCN113861034AEasy to synthesizeMild reaction conditionsLithium organic compoundsOrganic compound preparationBenzoic acidOrtho position
The invention discloses a preparation method of 2-fluoro-3-nitrobenzoic acid (II), which comprises the following steps: carrying out fluorine ortho-lithiation, dry ice carbonyl insertion reaction and acidic hydrolysis on 2-fluoronitrobenzene (I) by utilizing lithium diisopropylamide or tert-butyl lithium under a low-temperature condition to prepare the target product 2-fluoro-3-nitrobenzoic acid. The method has the advantages of mild reaction conditions, no need of transition metal catalysis, good selectivity, high yield, simple and efficient process, environmental protection, economy and suitableness for large-scale preparation;.
Owner:ZHEJIANG UNIV OF TECH
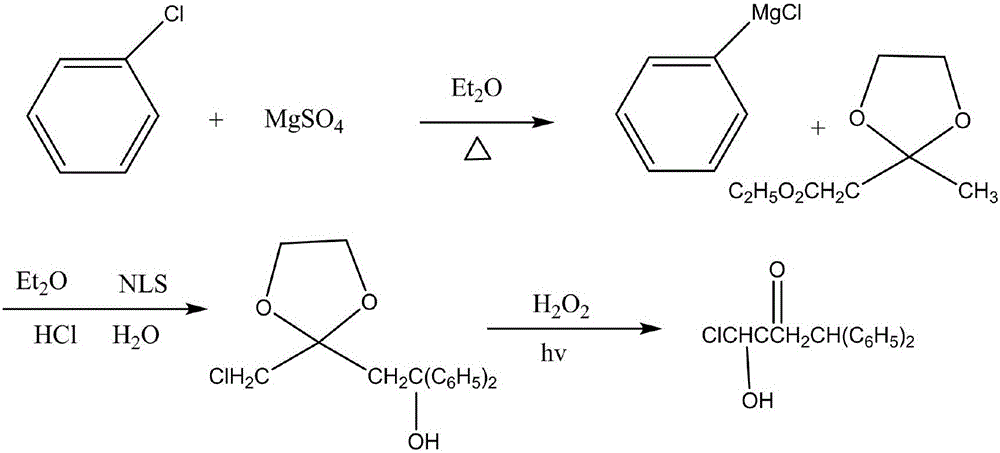
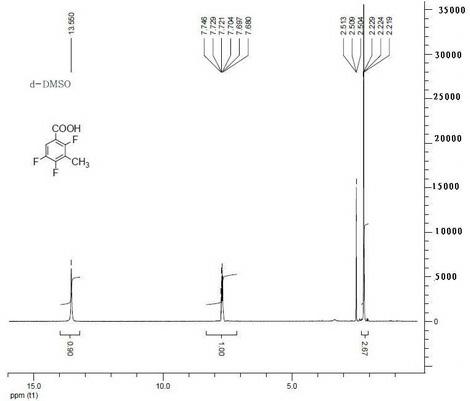
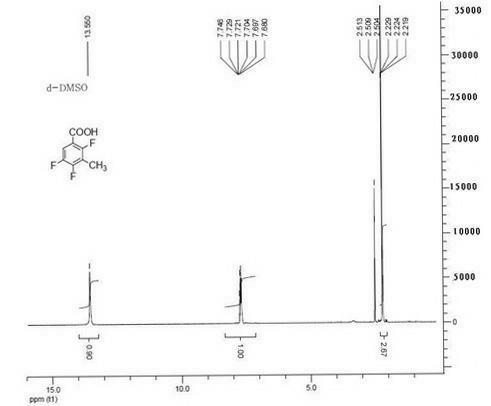
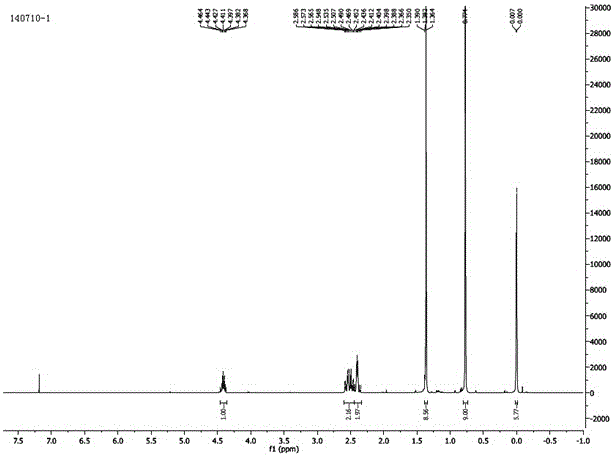
Synthesis method for 1-chloro-1-hydroxyl-4,4-diphenyl butyl-2-ketone
InactiveCN105753679AEasy to makeEasy to operatePreparation from heterocyclic compoundsSynthesis methodsTert-Butyllithium
The invention discloses a synthesis method for 1-chloro-1-hydroxyl-4,4-diphenyl butyl-2-ketone, and belongs to the field of organic synthesis techniques.For the defects that an existing synthetic route of 1-chloro-1-hydroxyl-4,4-diphenyl butyl-2-ketone is long, the total yield is very low and is no more than 21%, tert-butyllithium is used, danger exists, enlarged production is not easy to achieve, and extraction is not easy to achieve, the high-yield and safe synthesis method for 1-chloro-1-hydroxyl-4,4-diphenyl butyl-2-ketone is supplied.
Owner:CHANGZHOU UNIV
Method for preparing 2,4,5- trifluoro-3-methyl benzoic acid
ActiveCN102531887AReasonable designHigh yieldOrganic compound preparationCarboxylic compound preparationEthylenediamineBenzoic acid
The invention provides a method for preparing 2,4,5- trifluoro-3-methyl benzoic acid. The method is characterized in that 2,4,5-trifluorobenzoic acid reacts with mixed alkali under the condition of an organic solvent so as to obtain metal salt, of which the carboxylic group and the C3 site are substituted; mixed alkali comprises one of n-butyllithium or tert-butyllithium or lithium isopropoxide, one of potassium tert-butoxide or sodium methylate or potassium methoxide and one of 2,2,6,6-tetramethyl-piperidine or diisopropylamine or tetramethyl ethylenediamine or 2-hydrosulfuryl benzothiazole in a mixing manner, and organic solvent is alcohol ether solvent; metal salt reacts with methylating agents, and a substitution reaction is performed at the benzene ring C3 site so as to obtain 2,4,5- trifluoro-3-methyl benzoic acid; and the reaction temperature ranges from subzero 80 to 20 DEG C. The method has the advantages of high reaction temperature and low cost, and is easy for industrialized production.
Owner:锦州三溢科技有限公司
A kind of preparation method of (r)-tert-butyldimethylsiloxy-glutaric acid monoester
ActiveCN104370953BChiral naturalGood chiralityGroup 4/14 element organic compoundsHydroxybutyric acidGlutaric acid
The invention discloses a rosuvastatin intermediate (R)-tert butyl dimethyl siloxy-glutaric acid monoester preparation method, which belongs to the field of medicine. The method is as follows: compound (S)-4-halogen-3-hydroxy butyric acid ethyl ester is taken as a raw material for ester exchange to obtain other (S)-4-halogen-3-hydroxy butyric ester, (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate is obtained by protection of the (S)-4-halogen-3-hydroxy butyric ester, and by condensation of the (S)-4-halogen-3-tert butyl dimethyl siloxy benzyl butyrate and chloroformate in the presence of magnesium, tert butyl lithium and n-butyl lithium, (R)-tert butyl dimethyl siloxy-glutaric acid ester benzyl ester is obtained, and the (R)-tert butyl dimethyl siloxy-glutaric acid monoester is obtained by catalytic hydrogenation deprotection. Compared with the original process, the raw material of the process is chiral, natural, needs no resolution and induction and no enzyme hydrolysis to produce the chiral center, so that the product chirality is better. Compared with the original process, enzymes are already-industrialized enzymes, the raw material is easily obtained, reaction conditions are relatively mild, and the process is easy to industrialize. Compared with the original process, the process is shorter and less in three wastes.
Owner:ZHEJIANG LEPU PHARMA CO LTD
Synthetic method of 1,4-dialkylthiobenzene and its halides
InactiveCN106380431BImprove securityImprove maneuverabilityThiol preparationHydropoly/poly sulfide preparationAlkaneBenzene
The invention relates to a synthetic method for 1,4-dithioalkyl benzene and halide thereof. According to the method, 4-bromothiophenol is used as a raw material and reacts with alkyl bromide to synthesize 4-bromo-1-thioalkyl benzene, and then a reaction is carried out to synthesize 1,4-dithioalkyl benzene; or 4-bromothiophenol successively reacts with n-butyl lithium, sulfur powder and acid to synthesize 1,4-benzenedithiol, and then1,4-benzenedithiol reacts with alkyl bromide to synthesize 1,4-dithioalkyl benzene; and 1,4-dithioalkyl benzene can be further halogenated. The synthetic method provided by the invention does not need flammable and combustible t-butyl lithium, so the synthetic method has better security and controllability, is favorable for large-scale industrial production, reduces production cost and is environment friendly.
Owner:XIANGTAN UNIV
Metal sulfide and graphene composite material counter electrode and its preparation method and application
ActiveCN103903861BImprove conductivityImprove catalytic performanceLight-sensitive devicesFinal product manufactureMetallic sulfideSolar cell
The invention relates to a counter electrode made of two-dimensional metal sulfide and graphene composite materials, a preparation method of the counter electrode and application of the counter electrode in dye sensitization solar cells. Metal sulfide and graphene composites are loaded on a conductive substrate for preparation. The metal sulfide and graphene composites are dissolved in a solution and are deposited to form a film after being filtered, compressed film plating is carried out on the conductive substrate, drying is carried out, and then the obtained counter electrode is cooled to be at a room temperature, wherein the mass ratio of the metal sulfide to graphene is 20:1 to 20, and the mass ratio of sulfide to tert-butyllithium is 1:5 to 50. Binding agents do not need to be added, and accordingly high-temperature impurity removing is not needed, and the appearance and the structure of the materials can be well maintained. Compared with the material of a Pt counter electrode, the materials are richly reserved in nature, and industrial production can be carried out on a large scale. Compared with materials of other counter electrodes, the materials are easy and convenient to prepare and excellent in catalytic performance, and accordingly the two-dimensional metal sulfide and graphene composite materials have wide application prospects in the field of the dye sensitization solar cells.
Owner:NANKAI UNIV
A kind of preparation method of 2,5-disubstituted-1,4-terephthalaldehyde
ActiveCN112538007BHigh reactivityImprove Quantization EfficiencyOrganic compound preparationCarbonyl compound preparation by oxidationCarbenium ionOrganic synthesis
Owner:NINGBO POLYTECHNIC
Preparation method of microscale single-crystal ternary positive electrode material
ActiveCN109786672ASmall specific surface areaConcentrated particle size distributionElectrode manufacturing processesAir atmosphereManganese
The invention discloses a preparation method of a microscale single-crystal ternary positive electrode material. The preparation method comprises the steps of sanding and mixing raw materials; grinding nickel carbonyl, cobalt carbonyl, manganese carbonyl and tert-butyllithium in a grinding machine at a high speed according to a mole ratio being (0.2-0.6):(0.2-0.4):(0.2-0.5):(0.95-1.15), wherein the linear speed a of the grinding machine during working is equal to 20-35m / s, a grinding medium is tungsten particle, the particle size b is equal to 0.1-5 millimeter, and the grinding time is b*600 / ahours; and sintering a material, in which the material is sintered in an air atmosphere by a conventional round disc electric furnace to obtain the microscale single-crystal ternary positive electrode material, wherein the electric furnace power P is 1,500-3,000W, the electric furnace wire length L is 5-15 meters, and the sintering time is P*0.3 / L hours. The microscale single-crystal ternary positive electrode material synthesized by the process has the advantages of low specific area, concentrated grain distributed, high thermal stability and the like; and moreover, since the microscale single-crystal ternary positive electrode material has relatively low specific surface area, the contact degree of the material and an electrolyte is relatively low, and the cycle lifetime of the materialis greatly prolonged compared with a conventional spherical agglomeration material.
Owner:CHINA ELECTRONIC TECH GRP CORP NO 18 RES INST
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com