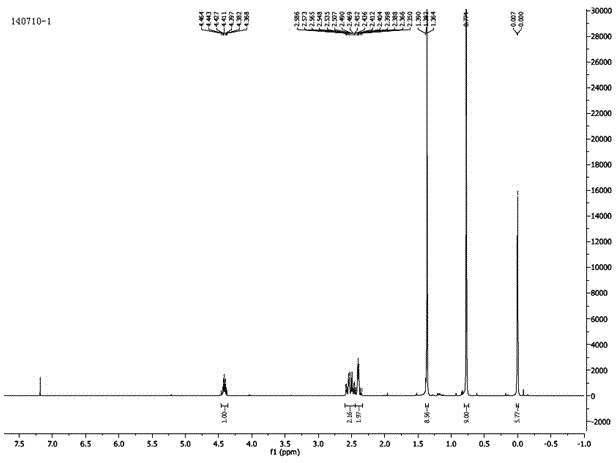
(R)-tert-butyl dimethyl siloxy-glutaric acid monoester preparation method
A technology of tert-butyldimethylsilyloxy and glutaric acid monoester, which is applied in chemical instruments and methods, compounds of elements of Group 4/14 of the periodic table, organic chemistry, etc., and can solve problems such as low yields , to achieve the effects of easy-to-obtain raw materials, good chirality of products, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0073] Preparation of Example 1 Compound IV:
[0074] In the reaction flask, add compound V, ethyl (S)-3-hydroxy-4-bromobutyrate (20.5g), benzyl alcohol (12g), toluene 150ml, YR-LIP (0.1g), heat to 70~ 80°C, 8 hours, TLC monitoring, after the reaction is complete, cool to room temperature, filter, recover the enzyme, and concentrate under reduced pressure to obtain a brown oily substance. The yield of (S)-benzyl 3-hydroxy-4-bromobutyrate (IV) was 96%, and the purity was 97% by GC.
example 2
[0075] The preparation of example 2 compound IV:
[0076] In the above example 1, YR-LIP Novozymes lipase was 4350.2g, and a brown oil was obtained. The yield of (S)-benzyl 3-hydroxy-4-bromobutyrate (IV) was 92%, and the purity was GC93%.
example 3
[0077] Preparation of Example 3 Compound IV:
[0078] In the reaction flask, add compound V, ethyl (S)-3-hydroxyl-4-bromobutyrate (20.5g), p-toluenesulfonic acid (0.3g), toluene, benzyl alcohol (10g), and heat 150ml of toluene to Reflux, react for about 6 hours, cool to normal temperature, wash with baking soda water, wash with brine, concentrate to remove toluene, and obtain oily product (S)-benzyl 3-hydroxy-4-bromobutyrate. The yield is 92%, and the GC purity is 93.5% %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com