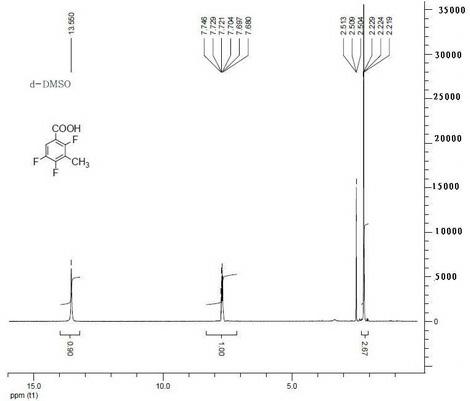
Method for preparing 2,4,5- trifluoro-3-methyl benzoic acid
A technology of methyl benzoic acid and trifluorobenzoic acid, which is applied in two fields, can solve the problems of high price and achieve the effects of low cost, high product yield and reasonable design
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1, a kind of preparation method of 2,4,5-trifluoro-3-methylbenzoic acid, its steps are as follows:
[0016] (1) 2,4,5-trifluorobenzoic acid is reacted with a mixed base in an organic solvent to obtain a metal salt with both carboxyl and C3 positions substituted; the reaction temperature is -80°C; 2,4,5-trifluorobenzoic acid The mass ratio of benzoic acid and mixed base is 1:1.5; Described mixed base is made of n-butyl lithium, a kind of in tert-butyl lithium or isopropyl lithium, potassium tert-butoxide, sodium methylate or potassium methylate One, and a mixed composition of 2,2,6,6-tetramethylpiperidine, diisopropylamine, tetramethylethylenediamine, and 2-mercaptobenzothiazole, and the three in sequence The mass ratio is 1:0.2:5; the organic solvent is alcohol ether solvent;
[0017] (2) React the metal salt with a methylating reagent at a reaction temperature of -80°C; a substitution reaction occurs at the C3 position of the benzene ring to obtain 2,4,5-tr...
Embodiment 2
[0018] Embodiment 2, a kind of preparation method of 2,4,5-trifluoro-3-methylbenzoic acid, its steps are as follows:
[0019] (1) 2,4,5-trifluorobenzoic acid reacts with a mixed base in an organic solvent to obtain a metal salt with both carboxyl and C3 positions substituted; the reaction temperature is 20°C; 2,4,5-trifluorobenzene The mass ratio of formic acid to the mixed base is 1:3; the mixed base is composed of one of n-butyl lithium, tert-butyl lithium or isopropyl lithium, one of potassium tert-butoxide, sodium methylate or potassium methylate species, and a mixture of 2,2,6,6-tetramethylpiperidine, diisopropylamine, tetramethylethylenediamine, and 2-mercaptobenzothiazole, the mass of the three in sequence The ratio is 1: 5: 0.2; the organic solvent is alcohol ether solvent;
[0020] (2) React the metal salt with a methylating reagent at a reaction temperature of 20°C; a substitution reaction occurs at the C3 position of the benzene ring to obtain 2,4,5-trifluoro-3-met...
Embodiment 3
[0021] Embodiment 3, a kind of preparation method of 2,4,5-trifluoro-3-methylbenzoic acid, its steps are as follows:
[0022] (1) 2,4,5-trifluorobenzoic acid reacts with a mixed base in an organic solvent to obtain a metal salt with both carboxyl and C3 positions substituted; the reaction temperature is -20°C; 2,4,5-trifluorobenzoic acid The mass ratio of benzoic acid and mixed base is 1:2.5; Described mixed base is made of n-butyl lithium, a kind of in tert-butyl lithium or isopropyl lithium, potassium tert-butoxide, sodium methylate or potassium methylate One, and a mixed composition of 2,2,6,6-tetramethylpiperidine, diisopropylamine, tetramethylethylenediamine, and 2-mercaptobenzothiazole, and the three in sequence The mass ratio is 1:1:1; the organic solvent is alcohol ether solvent;
[0023] (2) React the metal salt with a methylating reagent at a reaction temperature of -20°C; a substitution reaction occurs at the C3 position of the benzene ring to obtain 2,4,5-trifluor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com