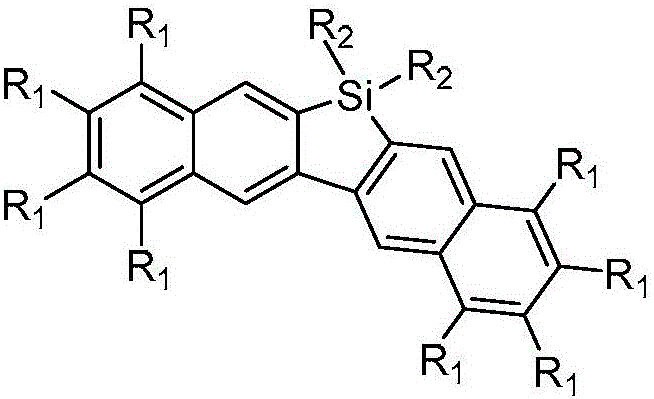
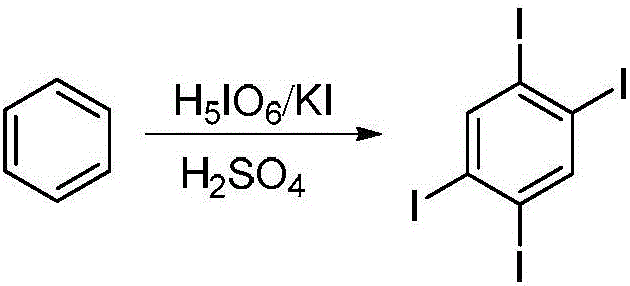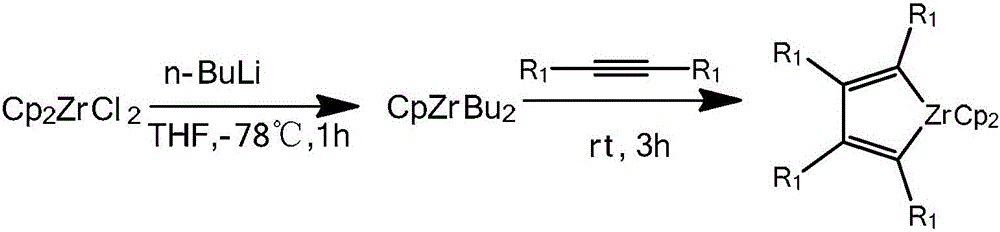Dinaphthosilole organic photoelectric functional material and synthesis method thereof
A technology of photoelectric functional materials and organic photoelectric materials, which is applied in the direction of luminescent materials, silicon organic compounds, organic chemistry, etc., can solve the problems of harsh synthesis conditions of silicon-containing materials and limited research reports, and achieve simple methods, high stereoselectivity, The effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1, the preparation of a dinaphthosilole organic photoelectric functional material, the steps are as follows:
[0024] Step 1, Synthesis of 1,2,4,5-tetraiodobenzene: Put 2.56g (11.2mmol) of periodic acid into a 250mL two-necked bottle, add 60mL of concentrated sulfuric acid while stirring, then slowly add 5.58g (33.6mmol ) KI, after 30 min, the temperature was lowered to 0° C., 1 mL (11.2 mmol) of benzene was slowly added thereto, and the reaction was carried out at room temperature for 24 hours. The reaction solution in the bottle was poured on ice, filtered, and washed with methanol to obtain a crude product. Recrystallized with 2-methoxyethanol to obtain 1,2,4,5-tetraiodobenzene with a yield of 71%.
[0025] Step 2, the synthesis of zirconium heterocyclopentadiene: under the protection of nitrogen, add 3.79g (13mmol) CpZr to the 200mL reaction tube 2 Cl 2 , and then added 50 mL of tetrahydrofuran, cooled to -78 ° C, added 10 mL (25 mmol) of n-butyllithiu...
Embodiment 2
[0032] Embodiment 2, the preparation of a dinaphthosilole organic photoelectric functional material, the steps are as follows:
[0033] Step 1, the synthesis method of 1,2,4,5-tetraiodobenzene is the same as that of Example 1.
[0034] Step 2, the synthesis of zirconium heterocyclopentadiene: under the protection of nitrogen, add 3.79g (13mmol) CpZr to the 200mL reaction tube 2 Cl 2 , and then added 50 mL of tetrahydrofuran, cooled to -78 ° C, added 10 mL (25 mmol) of n-butyllithium, and reacted at -78 ° C for 1 hour. Add 2.88 mL (20 mmol) of 4-octyne, return to room temperature, and stir for 3 hours to obtain zirconium cyclopentadiene.
[0035]Step 3. Synthesis of diiodonaphthalene b: Cool the zirconium heterocyclopentadiene to 0°C, add 1.18g (12mmol) cuprous chloride, 3.0ml (18mmol) DMPU and 10.56g (20mmol) 1,2,4 , 5-tetraiodobenzene, heat up to 50 ° C, react for 1 hour, add dilute hydrochloric acid to wash, add ether to extract, the organic phase is washed with saturated...
Embodiment 3
[0041] Embodiment 3, the preparation of a dinaphthosilole organic photoelectric functional material, the steps are as follows:
[0042] Step 1, synthesizing 1,2,4,5-tetraiodobenzene, the synthesis method is the same as that of Example 1.
[0043] Step 2, synthesizing zirconium heterocyclopentadiene, its synthetic method is identical with embodiment 2.
[0044] Step 3. Synthesis of diiodonaphthalene b: Cool the zirconium heterocyclopentadiene to 0°C, add 1.18g (12mmol) cuprous chloride, 6.336g (12mmol) DMPU, 1,2,4,5-tetraiodo Benzene, heat up to 50°C, react for 1 hour, add dilute hydrochloric acid to wash, add ether to extract, the organic phase is washed with saturated sodium bicarbonate solution, dried, filtered, and the solvent is removed by rotary evaporation. Separation and purification by column chromatography, using pure n-hexane as the eluent, yielded 1.83 g (yield 33.6%) of diiodonaphthalene b.
[0045] Step 4. Synthesis of binaphthalene b: under nitrogen protection,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com