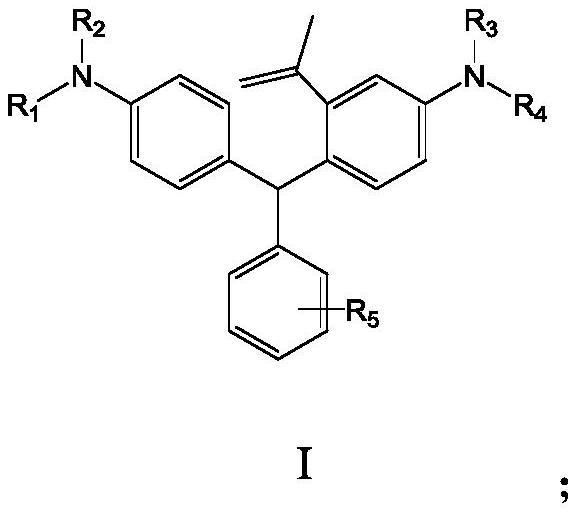
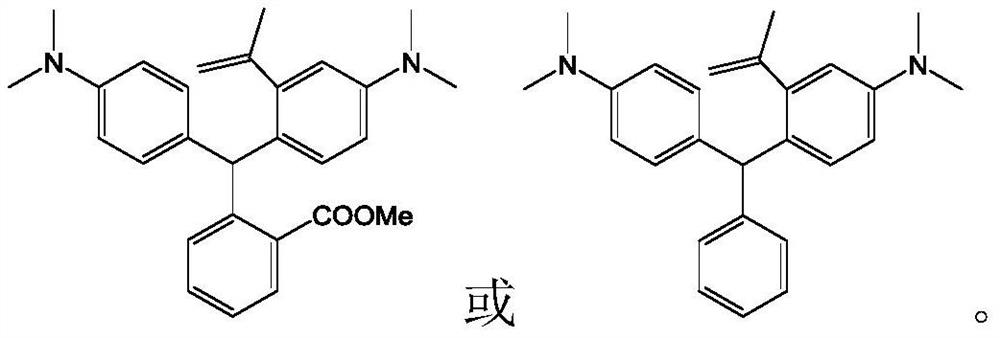
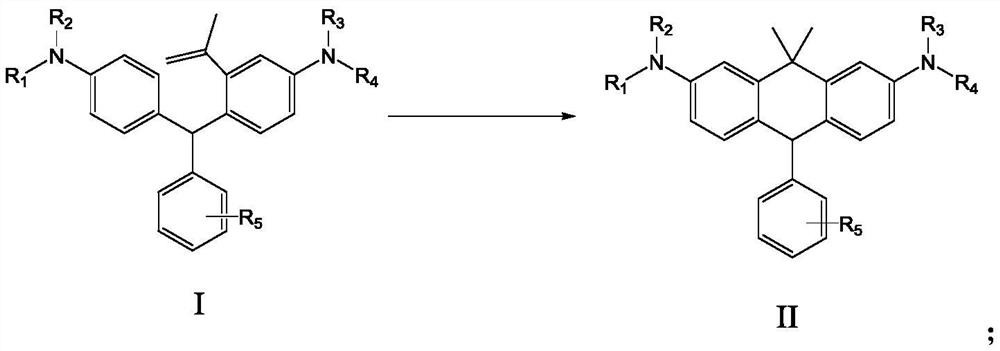
Benzene ring-containing compound as well as preparation method and application thereof
A compound and benzene ring technology, applied in the preparation of organic compounds, amino hydroxyl compounds, cyanide reaction preparation, etc., can solve the problem of increasing the production cost and operation difficulty of carbo-rhodamine compounds, unsatisfactory yield and long reaction route and other issues, to achieve the effect of facilitating large-scale production, simple product post-processing, and reducing the difficulty of reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0104]
[0105] 10 g (100 mmol) of compound 3-1 was added to 100 mL of THF and dissolved under stirring, and the temperature was lowered to -20°C under nitrogen protection. About 100 mmol of Grignard reagent 3-2 was slowly added dropwise to the solution of compound 3-1, during which the temperature was maintained not higher than -20°C, and the dropwise addition was completed in 30 min. The Grignard reaction was carried out for about 5 h under the condition of maintaining the temperature and stirring, quenched with about 100 mL of saturated ammonium chloride solution, and the pH value was adjusted to around 7. The mixture was extracted with ethyl acetate, and the organic phases were combined and dried over anhydrous sodium sulfate. After being concentrated under reduced pressure by rotary evaporation, use column chromatography (the stationary phase is 200-300 mesh silica gel powder, the eluent is n-hexane / ethyl acetate mixture, and the volume ratio is 10:1 to 2:1 according t...
Embodiment 2
[0115] 2-(3,6-bis(dimethylamino)-10,10-dimethyl-9,10-dihydroanthracene-9-yl)benzoic acid (2-(3,6-bis(dimethylamino)- Synthesis of 10,10-dimethyl-9,10-dihydroanthracen-9-yl)benzoic acid, compound 1-6)
[0116]
[0117] Under nitrogen protection, 20 g (100 mmol) of p-bromodimethylaniline was slowly added dropwise to 200 mL of THF containing 2.4 g (100 mmol) of magnesium chips, and a small grain of iodine (10 mg) was added, and the reaction was initiated by heating to 40-50 °C. , the dropwise addition time of p-bromodimethylaniline is about 30min. After the dropwise addition was completed, the temperature was kept at 40°C and the stirring was continued for about 2 hours until the magnesium chips basically disappeared. So far, the Grignard reagent shown in formula 1-2 is prepared.
[0118] 16.4 g (100 mmol) of methyl o-aldehyde benzoate was added to 100 mL of THF and stirred to dissolve, and the temperature was lowered to -20° C. under nitrogen protection. About 100 mmol of ...
Embodiment 3
[0121] Synthesis of Compounds 2-6
[0122]
[0123]Under nitrogen protection, 20g (100mmol) of p-bromodimethylaniline was slowly added dropwise to 100mL of THF containing 2.4g (100mmol) of magnesium chips, and a small particle of iodine (about 10mg) was added. Reaction, the dropwise time of p-bromodimethylaniline is about 30min. After the dropwise addition was completed, the temperature was kept at 40°C and the stirring was continued for about 4 hours until the magnesium chips basically disappeared. So far, the Grignard reagent shown in formula 2-2 is prepared.
[0124] 10 g (100 mmol) of benzaldehyde was added to 100 mL of THF and stirred to dissolve, and the temperature was lowered to -20°C under nitrogen protection. About 100 mmol of the prepared Grignard reagent was slowly added dropwise to the methyl o-aldehyde benzoate solution, and the temperature was maintained at no more than -20° C., and the dropwise addition was completed in 30 min. The grating reaction was ca...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com