Substituted thiazolidinedicarbamate bismuth complexes and uses thereof
A technology of thiazolidine ammonia and complexes, which is applied in the field of anticancer drug research, can solve the problems of few antitumor cell strains, low compound stability, and low clinical application value, and achieve good uptake rate, strong anticancer activity, and good Effect of antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
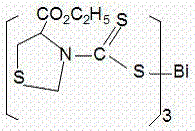
[0040] Preparation of bismuth thiazolidinedicarbamate (A)
[0041] Add 0.445g (5mmol) tetrahydrothiazole, 0.25g (6mmol) sodium hydroxide, and 15mL anhydrous methanol to a 50mL round bottom flask, stir and dissolve in a cold water bath at 0~5°C, then add 0.76g (10mmol) dropwise CS 2 , Low temperature magnetic stirring reaction for 2h, magnetic stirring reaction at room temperature for 4h.
[0042] 0.53g (1.7mmol) BiCl 3 Dissolve in 15 mL of anhydrous methanol, drop into the above reaction solution, stir at room temperature for 3 h, filter with suction, wash with anhydrous methanol, and dry in vacuo. The solid was recrystallized with dichloromethane and ethanol to obtain 0.94g of yellow powder solid, yield 80.3%, melting point: 137~138°C (carbonized to black).
[0043] Structural Characterization: Proton Magnetic Resonance (NMR) 1 HNMR), record the relevant signals of atoms under resonance as follows:
[0044] 1 HNMR (CDCl 3 ,400MHz),δ:3.184-3.215(6H,t,S-CH 2 ,J=6.4Hz),
...
Embodiment 2
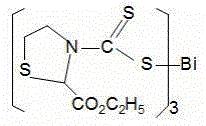
[0048] Preparation of 2-methylthiazolidinedicarbamate bismuth (B)
[0049] Add 0.516g (5mmol) 2-methylthiazolidine, 0.25g (6mmol) sodium hydroxide, and 15mL anhydrous methanol to a 50mL round bottom flask, stir and dissolve in a cold water bath at 0~5°C, then add 0.76g dropwise (10mmol) CS 2 , Low temperature magnetic stirring reaction for 2h, magnetic stirring reaction at room temperature for 4h.
[0050] 0.53g (1.7mmol) BiCl 3 Dissolve in 15mL of anhydrous methanol, drop into the above reaction solution, and stir at room temperature for 4h. A yellow precipitate precipitated, was suction filtered, washed with anhydrous methanol, and dried. The solid was recrystallized from acetonitrile to obtain 1.15 g of a yellow solid with a yield of 92.7% and a melting point of 139-140°C (carbonized to black).
[0051] Structural Characterization: Proton Magnetic Resonance (NMR) 1 HNMR), record the relevant signals of atoms under resonance as follows:
[0052] 1 HNMR (CDCl 3 ,400MH...
Embodiment 3
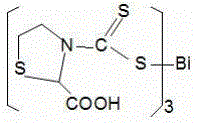
[0056] Preparation of 2-carboxythiazolidinedicarbamate (C)
[0057] Add 0.666g (5mmol) 2-thiazolidine formic acid, 0.40g (10mmol) sodium hydroxide, and 15mL anhydrous methanol into a 50mL round bottom flask, stir and dissolve in a cold water bath at 0~5°C, then add dropwise 0.76g ( 10 mmol) CS 2 , Low temperature magnetic stirring reaction for 2h, magnetic stirring reaction at room temperature for 4h.
[0058] 0.53g (1.7mmol) BiCl 3 Dissolve in 15mL of anhydrous methanol, drop into the above reaction solution, and stir at room temperature for 4h. Use 1:1 hydrochloric acid solution to adjust the pH value to 5-6, filter with suction, wash with deionized water, and dry in vacuum. 1.09 g of yellow solid was obtained, the yield was 78.4%, and the melting point was 136-138°C (carbonization decomposition).
[0059] Structural Characterization: Proton Magnetic Resonance (NMR) 1 HNMR), record the relevant signals of atoms under resonance as follows:
[0060] 1 HNMR (DMOS-d 6 ,4...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com