Method for joint production of strontium nitrate and barium nitrate from high-calcium strontianite and witherite
A joint production and witherite technology, applied in the direction of calcium/strontium/barium nitrate, etc., can solve the problems of environmental pollution, high cost, immature strontium salt technology, etc., and achieve the effect of reducing cost and improving utilization rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
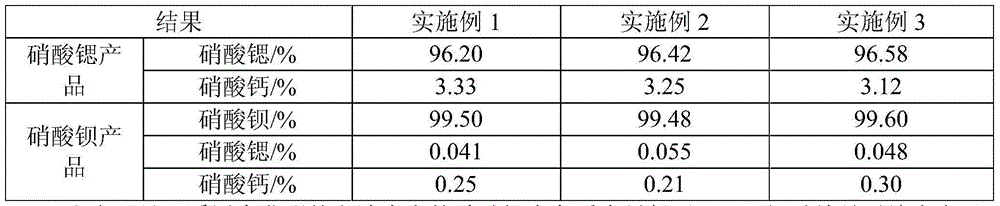
Embodiment 1
[0028] The present embodiment utilizes high-calcium strontite and witherite to jointly produce the method for strontium nitrate and barium nitrate, comprises the following steps:
[0029] a. Pulp preparation: respectively pulverize high-calcium strontite and witherite and mix with water to obtain strontium ore slurry and witherite slurry;
[0030] B, acidolysis: add the nitric acid of theoretical amount in the strontium ore pulp of step a gained, filter after reaction to obtain nitrate solution and strontium sulfate filter residue;
[0031] c. Impurity removal: add appropriate amount of hydrogen peroxide and activated carbon to the nitrate solution in step b to heat up the reaction, adjust the pH to neutral after the reaction, and finally filter to remove impurities in the nitrate solution;
[0032] d. Crystallization and separation: Evaporate the nitrate solution after crystallization and removal of impurities, and centrifuge to obtain strontium nitrate and mother liquor main...
Embodiment 2
[0046] The present embodiment utilizes high-calcium strontite and witherite to jointly produce the method for strontium nitrate and barium nitrate, comprises the following steps:
[0047] a. Pulp preparation: respectively pulverize high-calcium strontite and witherite and mix with water to obtain strontium ore slurry and witherite slurry;
[0048] B, acidolysis: add the nitric acid of theoretical amount in the strontium ore pulp of step a gained, filter after reaction to obtain nitrate solution and strontium sulfate filter residue;
[0049] c. Impurity removal: add appropriate amount of hydrogen peroxide and activated carbon to the nitrate solution in step b to heat up the reaction, adjust the pH to neutral after the reaction, and finally filter to remove impurities in the nitrate solution;
[0050] d. Crystallization and separation: Evaporate the nitrate solution after crystallization and removal of impurities, and centrifuge to obtain strontium nitrate and mother liquor main...
Embodiment 3
[0064] The present embodiment utilizes high-calcium strontite and witherite to jointly produce the method for strontium nitrate and barium nitrate, comprises the following steps:
[0065] a. Pulp preparation: respectively pulverize high-calcium strontite and witherite and mix with water to obtain strontium ore slurry and witherite slurry;
[0066] B, acidolysis: add the nitric acid of theoretical amount in the strontium ore pulp of step a gained, filter after reaction to obtain nitrate solution and strontium sulfate filter residue;
[0067] c. Impurity removal: add appropriate amount of hydrogen peroxide and activated carbon to the nitrate solution in step b to heat up the reaction, adjust the pH to neutral after the reaction, and finally filter to remove impurities in the nitrate solution;
[0068] d. Crystallization and separation: Evaporate the nitrate solution after crystallization and removal of impurities, and centrifuge to obtain strontium nitrate and mother liquor main...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com