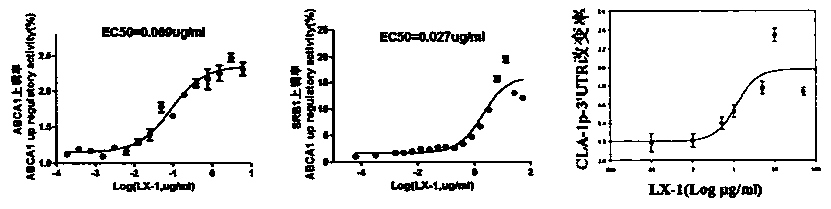
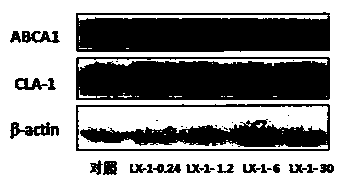
A group of 2-amido-3-alkoxy substituted pyridine compounds and their new applications
A compound and composition technology, applied in 2-amido-3-alkoxypyridine compounds, the preparation of the compound, the field of the pharmaceutical composition of the compound, to achieve good anti-atherosclerosis effect, broad The effect of the application foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] The preparation of embodiment 1N-(3-hydroxyl-2-pyridyl)-4-(1,3-dithio-2-cyclopentyl)benzamide (LX-1)
[0046] According to the operating method of Example 1, with 0.23g (0.001mol) 4-(1,3-dithio-2-cyclopentyl) benzoic acid and 0.11g (0.001mol) 2-amino-3-pyridinol Feed, to obtain LX-1 pure product 0.31g white solid, yield 97%. MS (ESI, m / z): 319[M]+, 1H NMR (500MHz, d6-DMSO) δ (ppm): 10.568 (s, 1H, NH-CO), 9.856 (s, 1H, -OH), 7.977(m, 2H, Ph-H), 7.655(m, 2H, Ph-H), 7.341(dd, 1H, J=8.0Hz, 1.5Hz, Py-6H), 7.224(dd, 1H, J=8.0 Hz, 5.0Hz, Py-4H), 5.843(s, 1H, SCHS), 5.337(t, 1H, J=5.0Hz, Py-5H), 3.57(m, 2H, CH2), 3.396(m, 2H, CH2)
Embodiment 2
[0047] The preparation of embodiment 2N-(3-hydroxyl-2-pyridyl)-4-trifluoromethoxybenzamide (LX-3)
[0048] According to the operating method of Example 1, 0.21g (0.001mol) 4-trifluoromethoxybenzoic acid and 0.11g (0.001mol) 2-amino-3-pyridinol were fed to obtain 0.25g of LX-3 pure product yellow Solid, 84% yield. MS (ESI, m / z): 299[M]+, 1H NMR (500MHz, d6-DMSO) δ (ppm): 8.151 (m, 1H, Ph-H), 7.960 (d, 1H, J=5.0Hz , Py-6H), 7.530(m, 1H, Ph-H), 7.326(d, 1H, J=7.5Hz, Py-4H), 7.203(m, 1H, NH-CO), 6.654(s, 1H, -OH), 5.337(t, 1H, J=5.0Hz, Py-5H),
Embodiment 3
[0049] The preparation of embodiment 3N-(3-hydroxyl-2-pyridyl)-4-iodobenzamide (LX-5)
[0050] According to the operation method of Example 1, feed intake with 0.25g (0.001mol) 4-iodobenzoic acid and 0.11g (0.001mol) 2-amino-3-pyridinol, obtain LX-5 pure product 0.36g white solid, the yield 90%. MS (ESI, m / z): 341[M]+, 1H NMR (500MHz, d6-DMSO) δ (ppm): 7.957 (s, 1H, NH), 7.924 (m, 2H, Ph-2H), 7.650 (s, 1H, Py-6), 7.323 (m, 2H, Ph-2H), 7.199 (s, 1H, OH), 5.337 (m, 2H, Py-4, 5H),
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com