The use of a group of carbamoylbenzenesulfonyl compounds
A carbamoylbenzenesulfonyl compound technology, applied in the pharmaceutical field, can solve the problems of lack of specific atherosclerotic drugs, severe side effects, etc., and achieve the effects of good anti-atherosclerosis effect, unique mechanism of action and novel structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
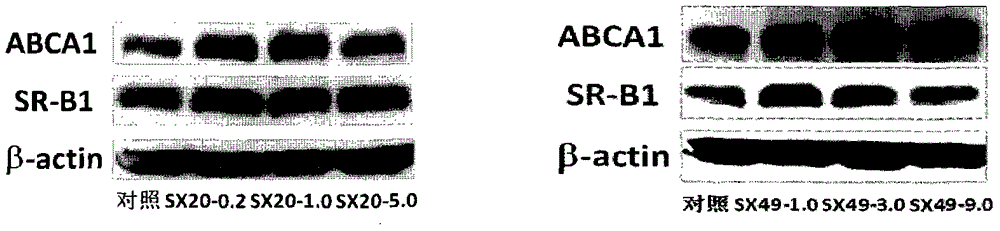
[0046] Example 1 The activity of the compound of the present invention on ABCA1 up-regulation screening model
[0047] ABCA1-LUC HepG2 cells in 5×10 4 Inoculate each well in a 96-well cell culture plate. After the cells adhere to the wall for about 6 hours, remove the serum-containing medium, gently rinse the cells once with PBS, and add serum-free MEM-EBSS medium to each experimental well. 200 μl, and then add 2 μl of the compound sample to be tested (final concentration 2.5 or 10 μg / ml), and the well with the final concentration of 0.1% DMSO medium is used as a blank control. After continuing to culture at 37° C. and 5% CO 2 for 18-24 h, the plate was washed twice with PBS (200 μl / well), and the PBS was discarded. Cell lysate (20 μl / well) (Promega) was added, and after 15-30 min, the complete cell lysis was observed under a microscope, then luciferase (60 μl / well) was added, and the luciferase activity was immediately measured (read by microplate reader).
[0048] Use the ...
Embodiment 2
[0051] Example 2 The activity of the compound of the present invention on the CLA-1 upregulator screening model
[0052] CLAP-LUC HepG2 cells in 5×10 4 Cells / well were seeded in a 96-well cell culture plate. After the cells adhered to the wall for about 6 hours, the serum-containing medium was removed, and the cells were gently rinsed with PBS once. Serum-free MEM-EBSS was added to each experimental well for culture. Base 200 μl, and then add 2 μl of the compound sample to be tested (final concentration 2.5 or 10 μg / ml), and the wells with the final concentration of 0.1% DMSO medium are used as blank control. After continuing to culture at 37°C and 5% CO2 for 18 hours, the plate was washed twice with PBS (200 μl / well), and the PBS was discarded. Cell lysate (20 μl / well) (Promega) was added, and after 15-30 min, the cell cleavage was observed under a microscope, then luciferase (60 μl / well) was added, and the luciferase activity was immediately measured (read by microplate rea...
Embodiment 3
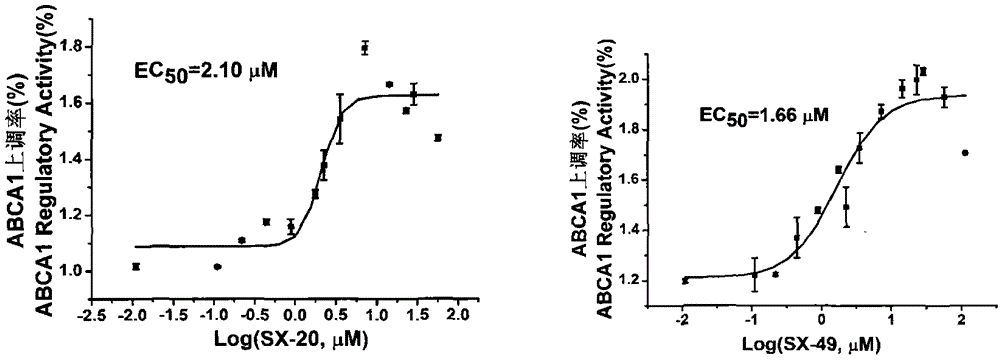
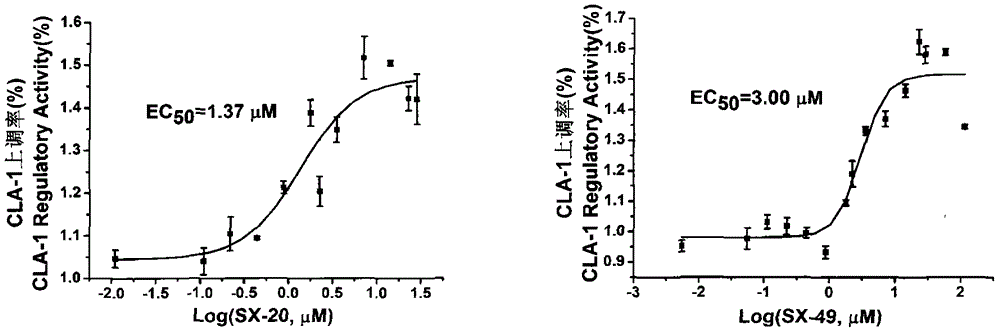
[0056] Example 3 EC50 Determination of Compounds of the Present Invention on ABCA1 and CLA-1 Up-regulation Model
[0057] The compound was dissolved in DMSO to make a 10 mg / ml stock solution. ABCA1p-LUC HepG2 and CLA-1p-LUC HepG2 cells were plated on a 96-well white plate (Costar), and the method was the same as before. Compounds diluted with serum-free RPMI1640 or MEM were made into a series of concentrations, 0.001-100 μmol / L, 200 μl / well. After 18-24h, the cell luciferase activity was measured, and the EC was calculated using Origin8.5 50 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com