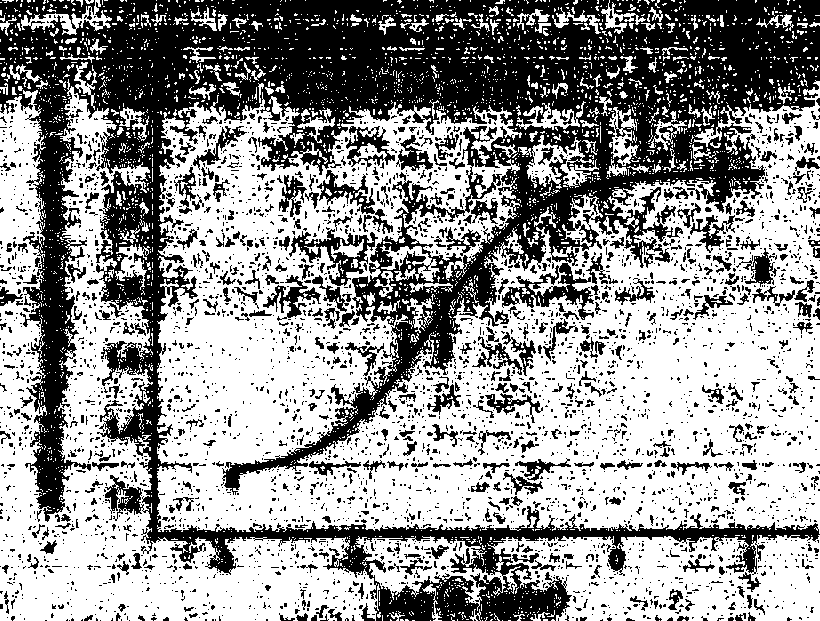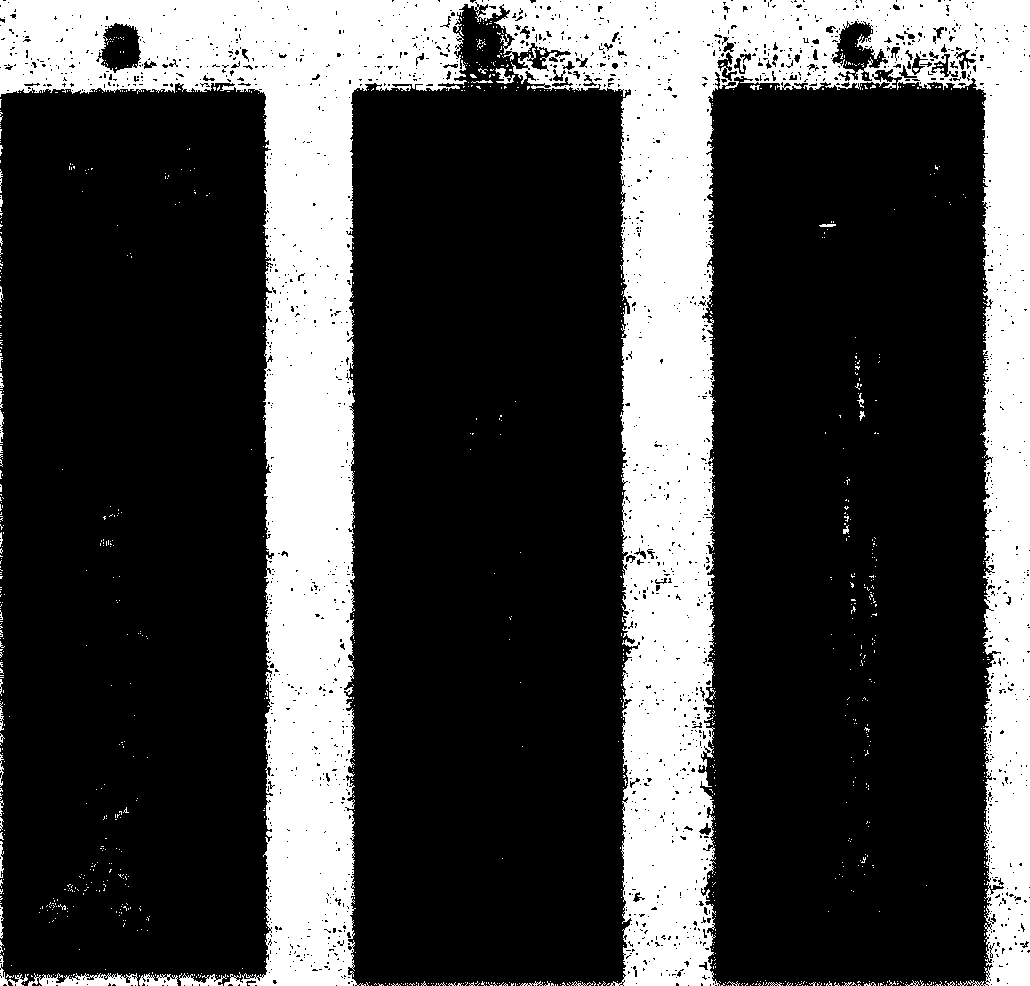Application of N-(4,5-dihydro-2-thiazole)-2-(4-methyl benzol phenoxy methyl) thiazole-4-formamide
A technology of methylphenoxymethyl and formamide, which is applied to the compound N--2-thiazole-4-carboxamide, the field of pharmaceutical compositions of the compound, has broad application prospects and good anti-atherosclerosis effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 The EC50 determination of the compound of the present invention on the ABCA1 up-regulation screening model
[0036] The compound was dissolved in DMSO to make a 10 mg / ml stock solution. ABCA1-LUC HepG2 cells in 5×10 4 Each cell / well was seeded in a 96-well cell culture plate. After the cells adhered to the wall for about 6 hours, the serum-containing medium was removed, and the cells were gently rinsed once with PBS. Compounds diluted with serum-free RPMI1640 or MEM were made into a series of concentrations, 0.001-100 μmol / L, 200 μl / well. Wells with a final concentration of 0.1% DMSO medium were used as blank controls. After continuing to culture at 37° C. and 5% CO 2 for 18-24 h, the plate was washed twice with PBS (200 μl / well), and the PBS was discarded. Add cell lysate (20 μl / well) (Promega), after 15-30min, observe under the microscope that the cell lysis is complete, add luciferase (60 μl / well), immediately measure luciferase activity (microplate read...
Embodiment 2
[0037] Example 2 Determination of the impact of the compound of the present invention on the expression level of ABCA1 protein
[0038] The effect of the compound on the expression of ABCA1 protein was detected by western blot method as before. HepG2 cells were plated in a six-well plate (Costar), and a drug-dosed group T61 (0.05, 0.24, 1.20, 6.0 μg / ml) and a negative control group (no sample to be tested) were set up. RIPA cell lysate was used to extract total cell protein, and BCA kit (Pierce) was used for protein quantification, followed by 10% SDS-PAGE electrophoresis, 30 μg protein per well. Concentration of each protein antibody used: ABCA1 (NB400-105; 1:500dilution; Novus Biologicals Inc., San Jose, CA); β-actin (1:5000; Invitrogen); peroxidase (HRP)-conjugated goat anti-rabbit IgG antibody (1:3000 dilution; Santa Cruz); peroxidase (HRP)-conjugated rabbit anti-mouse IgG antibody (1:3000 dilution; Santa Cruz). It was detected by chemiluminescence (ECL, Millipore) kit a...
Embodiment 3
[0039] Embodiment three in apoE - / - Pharmacodynamic evaluation in mice
[0040] apoE - / - Establishment of mouse arteriosclerosis model
[0041] apoE - / - Mice, 7 weeks old, were fed with common feed for one week;
[0042] apoE - / - The mice were weighed, randomly grouped, and divided into 4 groups (model group, drug administration group, negative control group), with 6-8 mice in each group;
[0043] From the age of 8 weeks, the model group and the treatment group were fed with high-fat feed, and the negative control group continued to be fed with common feed;
[0044] T61 (20mg / Kg) was administered by intragastric administration; the negative control group and the model group were given sodium carboxymethyl cellulose solution, administered by intragastric administration; administration for 8 weeks;
[0045] 8 weeks of apoE will be administered - / - The mice were fasted for 6 hours; the eyeballs were removed to collect blood, and the blood was collected in EP tubes rinsed w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com