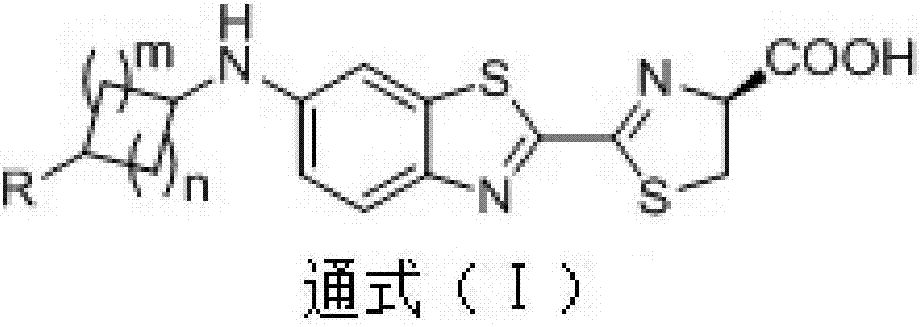
A cycloalkyl monosubstituted aminofluorescein compound and its preparation method and application
A technology of cycloalkyl mono- and compound, which is applied in the field of cycloalkyl mono-substituted amino luciferin compound and its preparation, can solve problems such as the influence of luminescent properties, and achieve the effects of good absorption in vivo, easy transmembrane, and long luminescent time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0041] [Example 1: Preparation of (S)-4-((2-(4-carboxy-4,5-dihydrothiazol-2-yl)benzothiazole-6-(cyclohexyl)amino (L1)]
[0042] Preparation of intermediate 2-cyano 6-cyclohexane alkylated aminobenzothiazole (1-1)
[0043] Dissolve 2-cyano 6-aminobenzothiazole (50mg, 0.28mmol) in 2mL of acetic acid, stir for five minutes, if not dissolved, then add cyclohexanone (1mL, mmol) and stir for 5 minutes, then add cyano Sodium borohydride (62mg, 1mmol) was added, and a large number of bubbles were generated. When the reaction solution presented a clear orange-red solution, ethyl acetate was added to the reaction solution, stirred for five minutes, and then saturated sodium carbonate solution was added in portions until the reaction No bubbles were generated in the solution. The reaction solution was washed with saturated sodium carbonate solution, extracted with ethyl acetate, the ethyl acetate layer was dried with anhydrous sodium sulfate, filtered, and then purified by a silica gel c...
example 3
[0057] [Example 3 Preparation of (S)-4-((2-(4-carboxy-4,5-dihydrothiazol-2-yl)benzothiazole-6-(cyclobutyl)amino (L3)]
[0058] Preparation of intermediate 2-cyano 6-cyclobutylated aminobenzothiazole (3-1)
[0059] Dissolve 2-cyano 6-aminobenzothiazole (50mg, 0.28mmol) in 2mL of acetic acid, stir for five minutes, if not dissolved, then add cyclobutanone (200μL,) and stir for 5 minutes, then add cyanoboron Sodium hydride (62mg, 1mmol) was added, and a large number of bubbles were generated. When the reaction solution presented a clear orange-red solution, ethyl acetate was added to the reaction solution, stirred for five minutes, and then saturated sodium carbonate solution was added in portions until the reaction solution No bubbles were generated in the medium. The reaction solution was washed with saturated sodium carbonate solution, extracted with ethyl acetate, the ethyl acetate layer was dried with anhydrous sodium sulfate, filtered, and then purified by a silica gel colu...
example 5
[0071] 【Example 5: Evaluation of the fat solubility of firefly luciferase substrate】
[0072] Three compounds L1, L2, and L3 with better activity were selected as the target compounds for further in-depth research. The target compound, D-luciferin, and aminoluciferin were calculated for their lipid solubility with Chembiodraw ultra 12.0 software, and CLogP was obtained. The results are shown in the following table:
[0073]
[0074] The results show that the CLogP of the target compound is larger than that of D-luciferin and aminoluciferin, so it has better fat solubility, which may give it a certain advantage in the process of cell transmembrane. [Example 6: Detection of the bioluminescent wavelength properties of the probe compound]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com