A kind of American white moth sumo‑abp‑dhc‑Cecropin A fusion protein and application thereof
A technology of sumo-abp-dhc-cecropina, American white moth, applied in the field of genetic engineering, can solve the problems of inability to realize large-scale production, high cost, complicated process, etc., and achieve obvious antifungal activity, low cost and high activity. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Cloning of Cecropin A cDNA
[0026] 1. Extract the total RNA of white moth.
[0027] Use the RNA extraction reagent (TIANGEN) to extract the total RNA of the fat body of the white moth pupa according to its operation manual, and identify its purity by formaldehyde-denatured agarose gel electrophoresis, which is greater than 95%, and its concentration is 720ng / µL, to meet the needs of use.
[0028] 2. Use Transgen cDNA reverse transcription kit to transcribe into first-strand cDNA.
[0029] 1) Primer design: According to the sequence alignment of Cecropin A in NCBI, Bombyx mori and Drosophila, degenerate primers were designed:
[0030] Cec-A1: 5'-ATGAATTTCNCAANAATNNNTNTNCTTCGT-3',
[0031] Cec-A2: 5'-NTATTTNCNAANGNTTTTTNCNGTACCCAG-3'.
[0032] 2) Reverse transcription: Add 3 μL of total RNA extract in sequence to a 1.5 mL Eppendorf tube treated with DEPC, Oligod (T) 18 (0.5 μg / μL) 1 μL, 2×TS Reaction Mix 10 μL, DEPC water 5 μL, TransScript RT / RI EnzymeMix...
Embodiment 2
[0034]Example 2 American white moth ABP- dHC -Cecropin A Escherichia coli in vitro expression
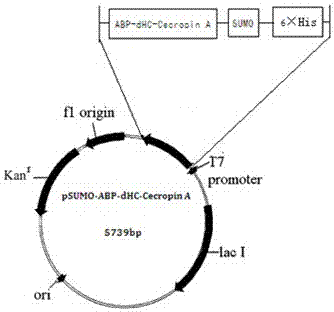
[0035] Construct the ABP-Cecropin A sequence of American white moth into the pET-SUMO plasmid region, such as figure 1 As shown, obtain pET-SUMO-ABP- dHC -Cecropin A, the specific construction method is as follows:
[0036] 1) According to the ABP obtained in Example 1- dHC - Cecropin A sequence design primers A1, A2,
[0037] A1 sequence: 5'-CAGGTGGAAGATCTTTAAGAAAATCG-3',
[0038] A2 sequence: 5'-CCCAAGCTTTTATTTTCTTAATGCTTTTGC-3';
[0039] where the 5' end of A1 has Stu1 Restriction site, the 5' end of A2 has HindⅢ Restriction sites.
[0040] Carry out PCR with American white moth DNA as a template, tap rubber to recover the PCR product, and recover the product for Stu1 and HindⅢ Enzyme digestion, SUMO vectors only use HindⅢ Enzyme digestion, T4 DNA Ligase ligation of the digested vector and PCR product, transformation of the ligation product E. coli In top10, t...
Embodiment 3
[0043] Example 3 American white moth ABP- dHC -Identification of antifungal activity of Cecropin A
[0044] 1) Anti-Candida albicans Canidia albicans activity identification
[0045] Determination of the antimicrobial peptide ABP- dHC -Cecropin A against Candida albicans Canidia Antibacterial activity of albicans. inoculation Canidia Albicans were cultured in liquid potato medium at 25°C and 200rpm for 2 hours, then added 1% of the bacteria to the melted potato agarose medium (35-50°C), mixed evenly and spread on sterile disposable plates at 10mL / dish . After solidification, punch a hole under sterile conditions, add the sample to be tested and place it in a 25°C incubator for 48 hours to observe the antibacterial activity. The results are shown in Image 6 A, has obvious antifungal activity.
[0046] 2) Resistance to Neurospora crassa Neurospora crassa activity identification
[0047] Determination of the antimicrobial peptide ABP- dHC -Cecropin A agains...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com