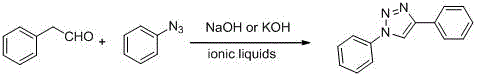A kind of preparation method of 1,4-disubstituted-1,2,3-triazole compound
A technology of triazole and disubstitution, which is applied in the field of synthesis of multi-substituted triazole compounds, can solve the problem of high yield and achieve the effects of simple catalytic system, mild reaction conditions and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
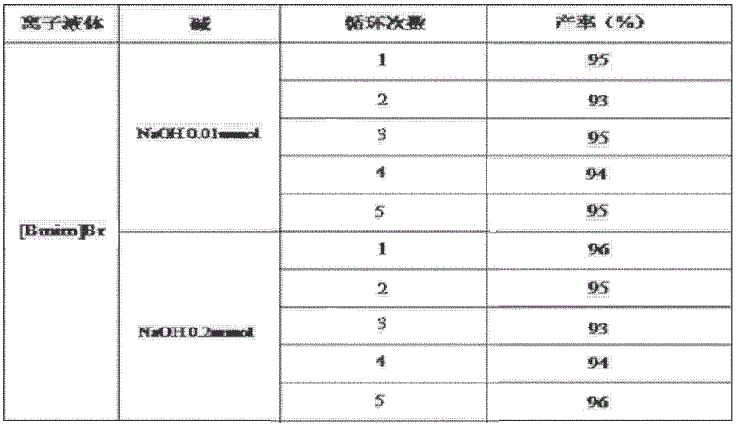
[0016] Weigh phenylacetaldehyde (120mg, 1mmol) and phenyl azide (119mg, 1mmol), measure ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF 4 ) 3mL, add NaOH (0.4mg, 0.01mmol), stir at room temperature, magnetically stir, TLC monitors the reaction, after 10min, the reaction is over, there is solid precipitation, ether extraction, drying, you can get 1,4-diphenyl-1H- 1,2,3-Triazole, the yield is 95%.
Embodiment 2
[0018] Weigh phenylacetaldehyde (120mg, 1mmol) and phenyl azide (119mg, 1mmol), measure ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF 4 ) 3mL, add NaOH (8mg, 0.2mmol), room temperature, magnetic stirring, TLC monitoring reaction, after 10min, the reaction is over, there is solid precipitation, ether extraction, drying, you can get 1,4-diphenyl-1H-1 , 2,3-triazole, yield 96%.
Embodiment 3
[0020] Weigh phenylacetaldehyde (120mg, 1mmol) and phenyl azide (119mg, 1mmol), measure ionic liquid 1-butyl-3-methylimidazolium tetrafluoroborate ([Bmim]BF 4 ) 3mL, add KOH (0.56mg, 0.01mmol), stir at room temperature, magnetically stir, TLC monitors the reaction, after 10min, the reaction is over, there is solid precipitation, ether extraction, drying, you can get 1,4-diphenyl-1H- 1,2,3-triazole, yield 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com