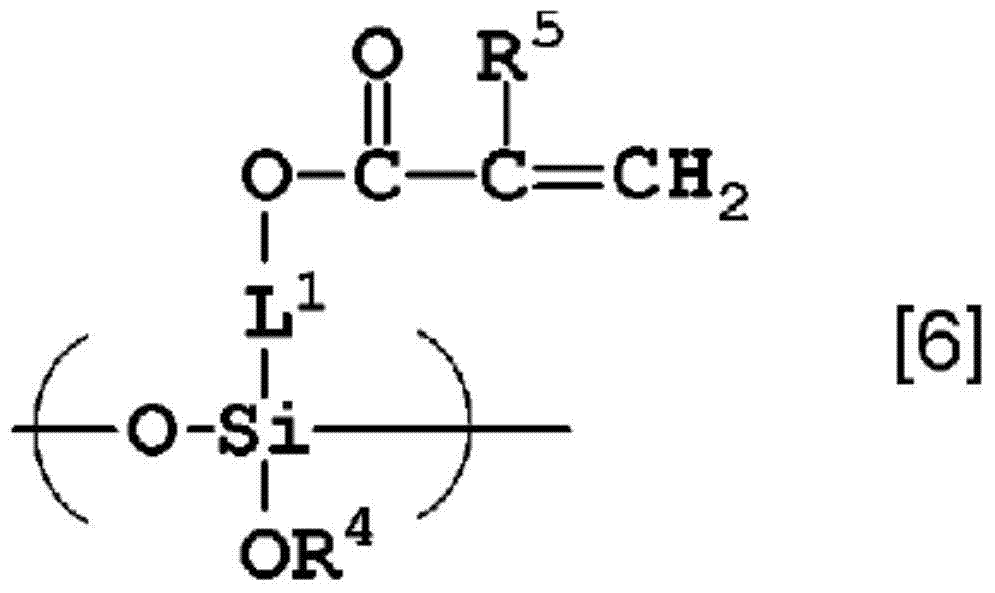
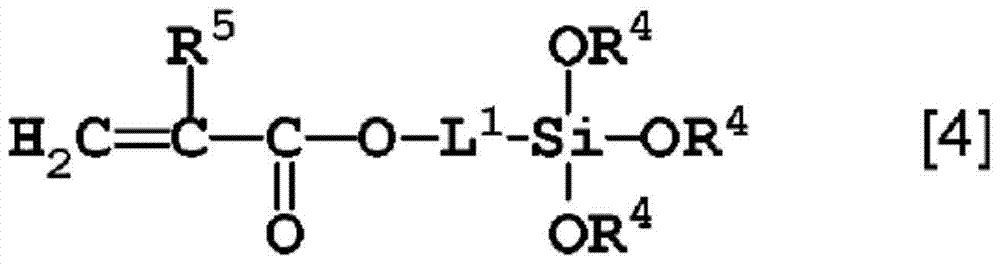Curable composition containing fluorine-containing hyperbranched polymer and siloxane oligomer
一种固化性组合物、硅氧烷低聚物的技术,应用在硬涂层的叠层体,硅氧烷低聚物及其制造领域,能够解决固化收缩大、玻璃表面没有密合性、不形成化学键等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0215] Hereinafter, examples will be given to explain the present invention more specifically, but the present invention is not limited to the following examples.
[0216] In addition, in the examples, the equipment and conditions used for sample preparation and physical property analysis are as follows.
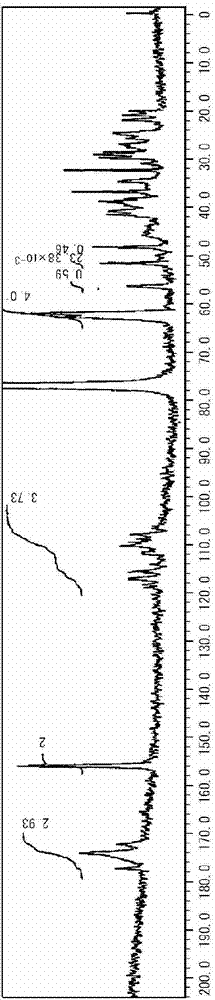
[0217] (1) 13 C NMR spectrum
[0218] Device: JNM-ECA700 manufactured by Nippon Electronics Data Corporation
[0219] Solvent: CDCl 3
[0220] Benchmark: CDCl 3 (77.0ppm)
[0221] (2) Gel Permeation Chromatography (GPC)
[0222] Device: HLC-8220GPC manufactured by Tosei Co., Ltd.
[0223] Column: Showa Denko Co., Ltd. Shodex (registered trademark) GPC KF-804L, GPC KF-805L
[0224] Column temperature: 40℃
[0225] Solvent: Tetrahydrofuran
[0226] Detector: RI
[0227] (3) Determination of glass transition temperature (Tg)
[0228] Installation: Photo-DSC 204 F1 Phoenix (registered trademark) manufactured by NETZSCH
[0229] Measurement conditions: under nitrogen atmosphere
[0230] Heating rate...
Synthetic example 1
[0272] [Synthesis Example 1] Production of fluorine-containing hyperbranched polymer (F-HBP)
[0273] MIBK54g was put into a 300 mL reaction flask, nitrogen was injected for 5 minutes while stirring, and heating was performed until the inner liquid refluxed (about 116°C).
[0274] In another 100 mL reaction flask, 9.0 g (20 mmol) of IPDUA as monomer A, 5.9 g (14 mmol) of C6FA as monomer B, 2.3 g (10 mmol) of MAIB as polymerization initiator C and 54 g of MIBK were added. While stirring, nitrogen was injected for 5 minutes to perform nitrogen replacement, and then cooled to 0°C with an ice bath.
[0275] To the refluxing MIBK in the above 300 mL reaction flask, the contents were dripped over 30 minutes from the above 100 mL reaction flask containing IPDUA, C6FA, and MAIB using a drip pump. After the dropwise addition, it was further stirred for 1 hour.
[0276] Next, after 35 g of MIBK was distilled off from the reaction liquid using a rotary evaporator, it was added to 450 g of hexan...
Synthetic example 2
[0278] [Synthesis Example 2] Production of siloxane oligomer (Si-OLG-1)
[0279] In a 100 mL reaction flask, 11.6 g of MPTES and 7.4 g of ethanol were added and stirred for 5 minutes. In this solution, a mixed solution of 0.04 g of oxalic acid [manufactured by Kanto Chemical Co., Ltd.], 3.2 g of water, and 7.4 g of ethanol separately prepared was added dropwise over 30 minutes. After the solution was stirred for 5 minutes, it was heated until the inner liquid refluxed (approximately 80°C) and stirred for 1 hour. The reaction mixture was cooled to 30°C, and an ethanol solution of Si-OLG-1 was obtained.
[0280] The weight average molecular weight Mw of Si-OLG-1 measured by GPC in terms of polystyrene was 1,200, and the degree of dispersion: Mw / Mn was 1.1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com