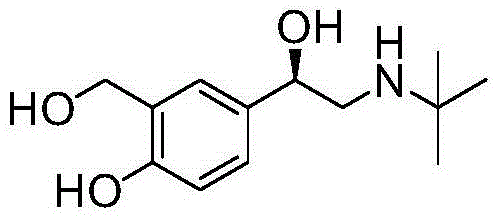
Asymmetric preparation method for (R)-salbutamol hydrochloride
A technology for salbutamol and hydrochloride, which is applied in the preparation of organic compounds, chemical instruments and methods, and the preparation of aminohydroxyl compounds. Cost of production, high yield, effect of avoiding splitting process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
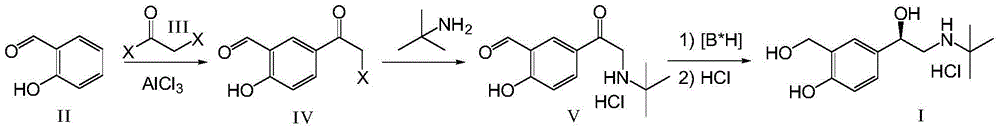
[0041] (1) Add 200g (0.15mol) of anhydrous aluminum trichloride and 150mL of dichloroethane to a 250mL three-necked flask, raise the temperature to 70°C, add dropwise 150mL of a dichloroethane solution of 66.1g (0.042mol) bromoacetyl chloride, After the dissolution was complete, 36.6g (0.03mol) of salicylaldehyde in 150mL of dichloroethane was dropped into the reaction solution at 70°C, and after the dropwise addition, the temperature was raised to 80°C for 36 hours of reaction. Slowly pour the reaction solution into 1.0kg of crushed ice under stirring, add 100mL of concentrated hydrochloric acid, separate the organic layer, extract the water layer with 200mL of dichloroethane, combine the dichloroethane layers, wash with water (300mLx2), and saturated salt Wash with water (300mL), dry over anhydrous sodium sulfate, and concentrate to obtain a yellow oil, which is crystallized with petroleum ether-dichloromethane, filtered, and dried to obtain 58g of white solid intermediate II...
Embodiment 2
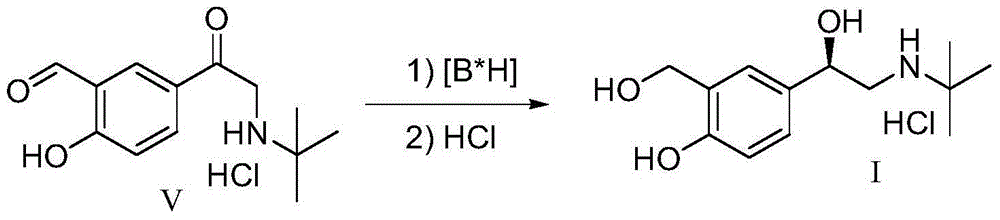
[0046] The intermediate V prepared in Example 1 above was used as a raw material. Add 95g (66.6mmol) boron trifluoride diethyl ether in 40mL tetrahydrofuran solution dropwise into 2.1g (55.5mmol) sodium borohydride suspension in 50mL tetrahydrofuran under the protection of 20 nitrogen, add 1.0g (4mmol) after heating to reflux for 0.5 hours A solution of (R)-diphenylprolinol in 10 mL of tetrahydrofuran was refluxed for 0.5 hours. Cool to 25°C, slowly add 10g (37mmol) intermediate V in 200mL tetrahydrofuran solution dropwise, and the dropwise addition is completed in about 3 hours. Concentrate under reduced pressure to about 50 mL of solvent, add 100 mL of 2N hydrochloric acid, extract with ethyl acetate (100 mL x 2), dry over anhydrous sodium sulfate, and concentrate to obtain (R)-salbutamol as a yellow solid. Dissolve the crude (R)-salbutamol in 100 mL of ethyl acetate, add dropwise 30 mL of 2M ethyl acetate hydrogen chloride solution, a large amount of white solid (R)-salbut...
Embodiment 3
[0048] The intermediate V prepared in Example 1 above was used as a raw material. Under the protection of nitrogen, 95g (66.6mmol) of boron trifluoride diethyl ether in 40mL of dioxane solution was added dropwise to 2.1g (55.5mmol) of sodium borohydride in 50mL of dioxane suspension, heated to reflux for 0.5 hours and then added 1.0 g (4mmol) (R)-diphenylprolinol in 10mL dioxane solution, and then refluxed for 0.5 hours. Cool to 25°C, slowly add 10g (37mmol) intermediate V in 200mL dioxane solution dropwise, and dropwise finish in about 3 hours. Concentrate under reduced pressure to about 50 mL of solvent, add 100 mL of 2N hydrochloric acid, extract with ethyl acetate (100 mL x 2), dry over anhydrous sodium sulfate, and concentrate to obtain (R)-salbutamol as a yellow solid. Dissolve the crude (R)-salbutamol in 100 mL of ethyl acetate, add dropwise 30 mL of 2M ethyl acetate hydrogen chloride solution, a large amount of white solid (R)-salbutamol hydrochloride precipitates, fi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap