Preparation method for dabigatran etexilate intermediate cyclocompound
A technology of dabigatran etexilate and cyclization products, which is applied in the field of preparation of dabigatran etexilate intermediate cyclization products, can solve problems such as low purity, difficult operation, and difficulty in meeting the strict requirements of anhydrous water limit, and achieve purity High, high yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
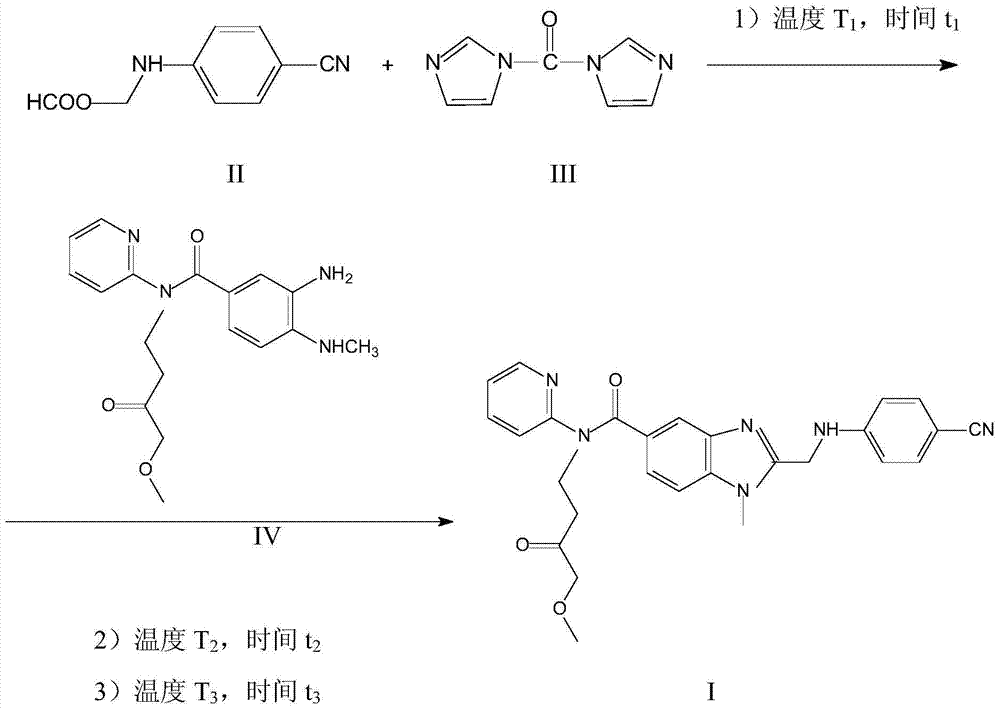
[0032] Add 1.77g (10mmol) of compound (II), 1.42g (10mmol) of compound (III), and 10ml of toluene, and heat up to 50°C for the first reaction for 1 hour. Add 3.42g (10mmol) of compound (IV) and raise the temperature to 60°C for the second reaction for 3 hours. The temperature was raised to 100° C. for the third reaction for 3 hours. The toluene was evaporated, and 5ml of ethanol was added to re-purify to obtain 4.30g of off-white powder cyclized product. Yield 84.6%, content 95.0%.
Embodiment 2
[0034] Add 1.77g (10mmol) of compound (II), 1.70g (12mmol) of compound (III), and 40ml of toluene, and heat up to 50°C for the first reaction for 1 hour. Add 4.10 g (12 mmol) of compound (IV) and raise the temperature to 60° C. for the second reaction for 3 hours. The temperature was raised to 100° C. for the third reaction for 3 hours. Toluene was evaporated, and 20ml of ethanol was added to re-purify to obtain 4.20g of off-white powder cyclized product. Yield 82.4%, content 94.8%.
Embodiment 3
[0036] Add 1.77g (10mmol) of compound (II), 1.42g (10mmol) of compound (III), and 10ml of heptane, and heat up to 30°C for the first reaction for 3 hours. Add 3.42g (10mmol) of compound (IV) and raise the temperature to 40°C for the second reaction for 6 hours. The temperature was raised to 60° C. for the third reaction for 6 hours. The heptane was distilled off, and 5 ml of methanol was added to re-purify to obtain 4.20 g of off-white powder cyclized product. Yield 81.9%, content 94.1%.
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap