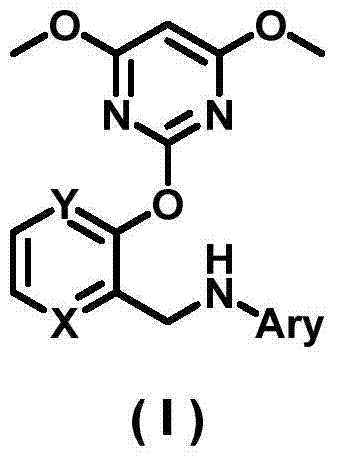
2-pyrimidinyloxy-N-arylazabenzylamine compound, preparation method, herbicide and application thereof
A technology of aryl azabenzylamine and pyrimidinyloxy, which is applied in the field of 2-pyrimidinyloxy-N-arylazazebenzylamine compounds, and can solve the problems of low universality, poor selectivity and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
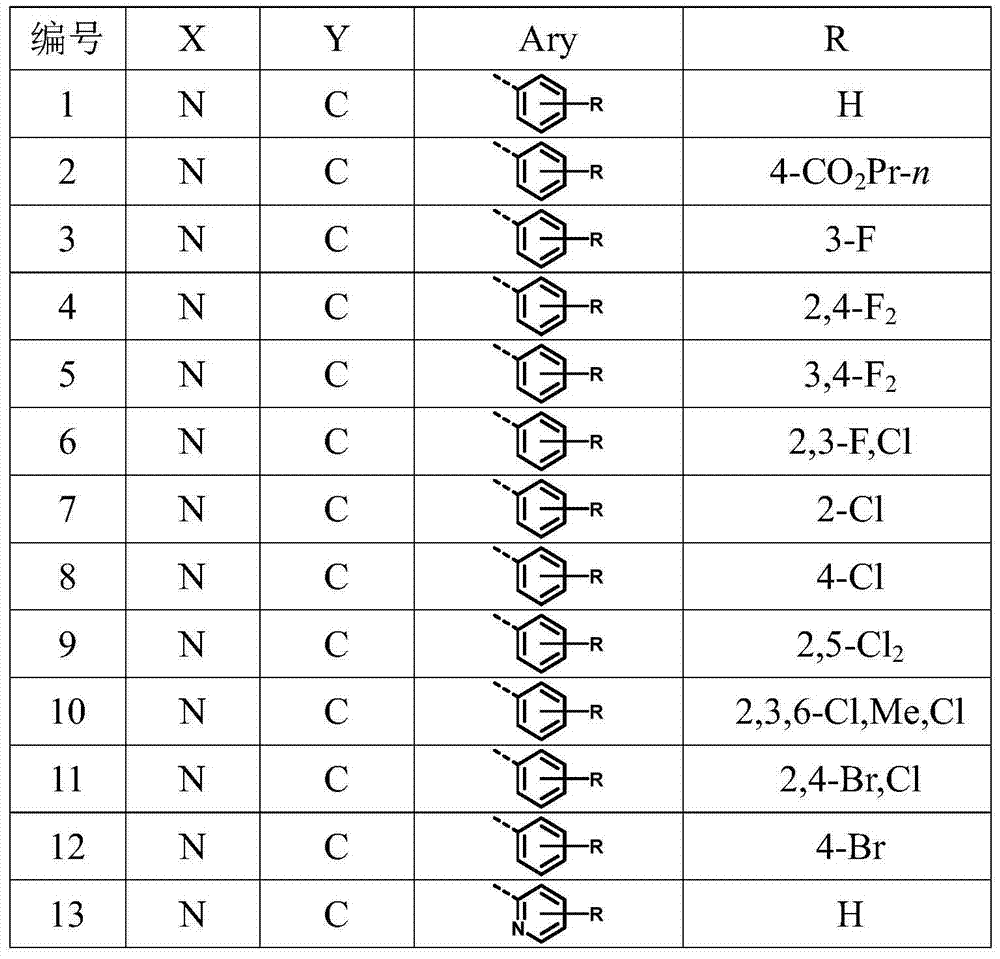
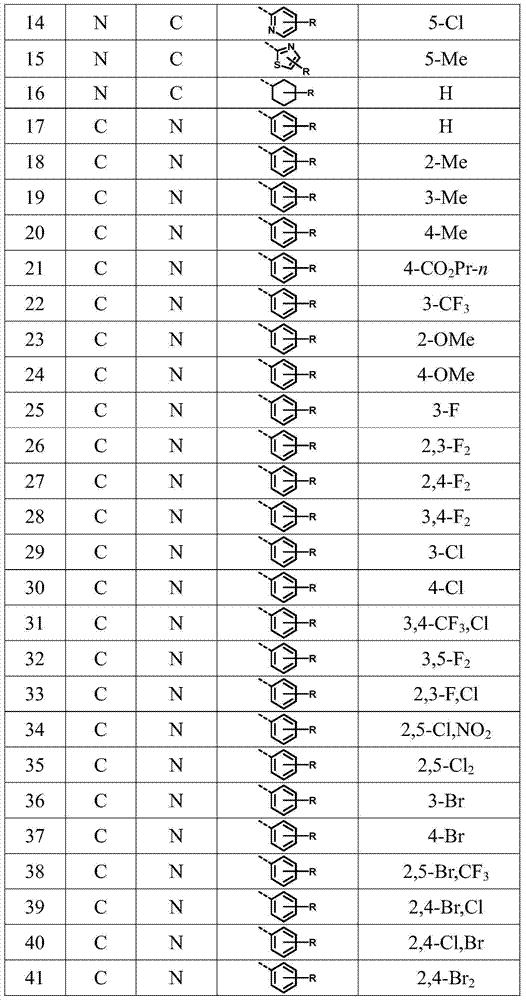
[0081] Examples of preparation methods of compounds 2-42 of the present invention Referring to Example 1, select corresponding reaction raw materials according to different products.
[0082] In Examples 43-47, all "%" refer to percentage by weight, "g a.i. / hm 2 "All refer to grams of active substance / ha.
Embodiment 1
[0083] Synthesis of embodiment 1 compound (1)
[0084]
[0085] 1) 2-[(phenylimino)methyl]-1-azabenzene-3-ol (g)
[0086] At 10-15°C, add aniline (3.97g, 42.64mmol) into a solution of 3-hydroxy-2-pyridinecarbaldehyde (f, 5.00g, 40.61mmol) in anhydrous methanol (80mL), stir for 1h to precipitate a yellow solid, The mixed system was left to stand for 5 h, filtered, and the yellow solid was washed with a small amount of anhydrous methanol (5 mL / time) precooled to below 5°C. The yellow solid was dried in vacuo to obtain the target compound 2-[(phenylimino)methyl]-1-azabenzene-3-ol (g, 7.36g, 92%).
[0087] 2) 2-[(phenylamino)methyl]-1-azabenzene-3-ol (h)
[0088] At 0-5°C, sodium borohydride (4.07g, 107.45mmol) was added in batches to 2-[(phenylimino)methyl]-1-azaphenol (g, 7.10g, 35.82mmol) and no In a suspension of water and methanol (150 mL), stirring was continued for 30 min. After the reaction, add saturated ammonium chloride solution (70mL) to the reaction system, sti...
Embodiment 2
[0092] n-Propyl N-[2-(4,6-dimethoxypyrimidinyloxy)-6-azabenzylamino]benzoate (2): white solid, mp: 110.5–111.6℃.ESI-MS(m / z):425[M+H] + ,447[M+Na] + . 1 H NMR (300MHz, DMSO) δ8.53–8.33 (m, 1H), 7.70 (d, J = 8.0Hz, 1H), 7.59 (d, J = 8.2Hz, 2H), 7.41 (s, 1H), 6.58 (d, J=8.2Hz, 2H), 6.00(s, 1H), 4.37(d, J=4.6Hz, 2H), 4.09(t, J=6.0Hz, 2H), 3.71(s, 6H), 1.64 (dd,J=13.8,6.8Hz,2H),0.92(t,J=7.1Hz,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com