A method for promoting extracellular expression of target protein and its application
A technology of target protein and protein, which is applied in the field of promoting the extracellular expression of target protein, can solve problems such as unfavorable fermentation regulation and industrial amplification, and achieves the improvement of the ratio of extracellular enzyme activity and production intensity, the improvement of production intensity, and the convenient regulation of enzyme production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Expression of Phospholipase C in E. coli BL21 (DE3)
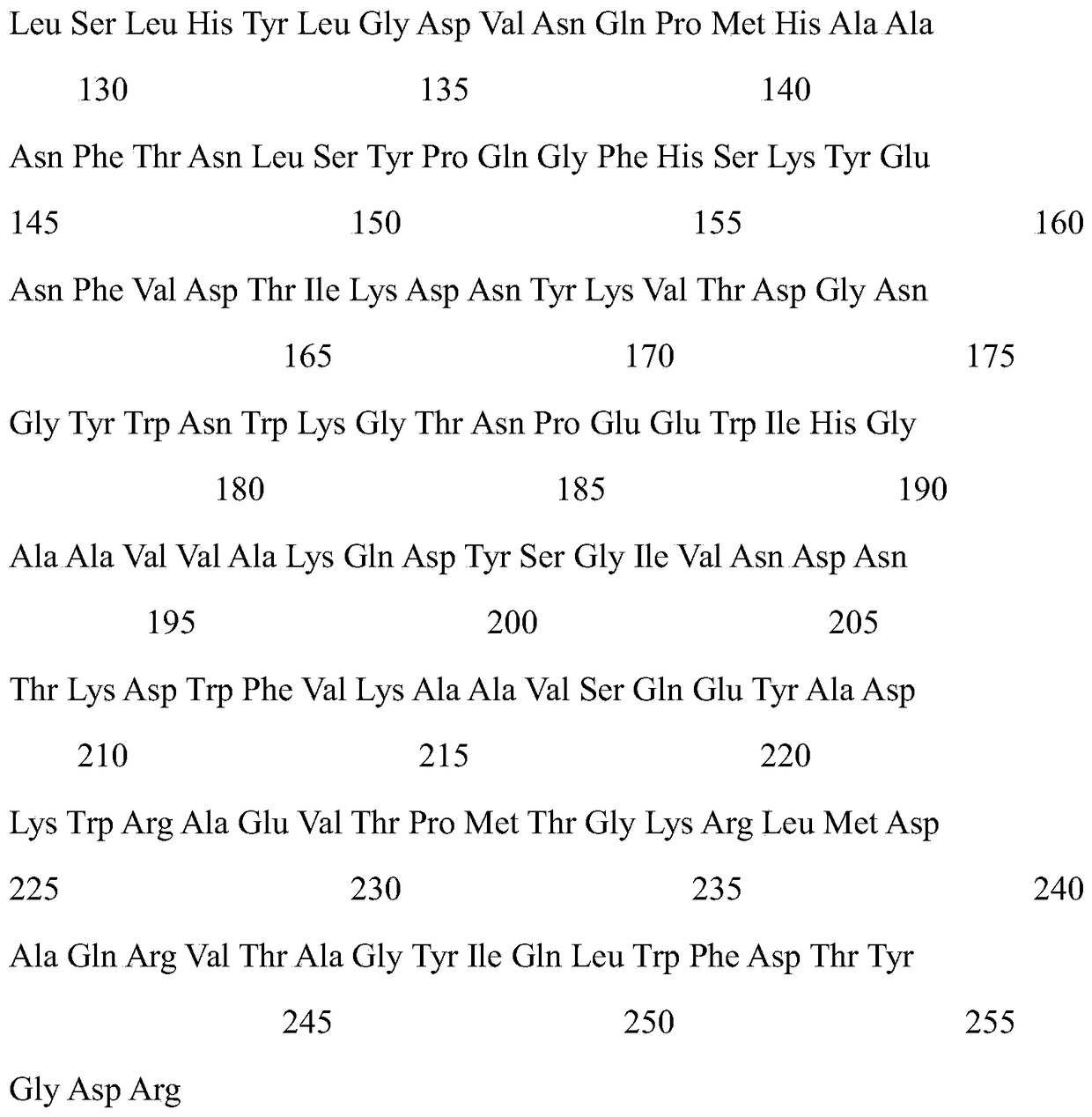
[0025] Construction of pETDuet-1-plc recombinant plasmid: pETDuet-1 plasmid (purchased from Novagen) and pMD18-T-plc carrying phospholipase C gene (amino acid sequence shown in SEQ ID NO.1) were subjected to Nco I, After double digestion with Hind Ⅲ, the digestion product was recovered by gel tapping, T4 ligase was ligated overnight at 16 °C, and the ligation product was transformed into E. coli JM109, which was coated on 100 μg·mL ampicillin -1 The LB agar plates were cultured at 37 °C for 8-10 h, and the transformants were selected with an ampicillin content of 100 μg·mL. -1 The LB liquid medium was cultured for 8-10h, and the plasmid was extracted to obtain the recombinant plasmid pETDuet-1-plc.
[0026] Expression and preparation of phospholipase C: The pETDuet-1-plc plasmid was transformed into E.coli BL21 (DE3) host bacteria, and the ampicillin content was 100 μg·mL -1 Cultured on LB agar plates at...
Embodiment 2
[0028] Example 2: Co-expression of xylanase and phospholipase C in E. coli 3L tank fermentation
[0029] Construction of pETDuet-1-plc-XynA recombinant plasmid: PETDuet-1-plc plasmid and pET20b(+)-XynA of the xylanase gene with NCBI number AGM16410 cloned into pET20b(+) were subjected to Nde I and Xho I Double-enzyme digestion, the digestion product was recovered by gel cutting, and then ligated with T4 ligase at 16°C overnight. The ligation product was transformed into E.coli JM109 competent cells, and the transformation product was coated with ampicillin with a content of 100 μg·mL -1 The LB agar plates were cultured at 37 °C for 8-10 h, and the transformants were selected with an ampicillin content of 100 μg·mL. -1 The LB liquid medium was cultured for 8-10h, and then the plasmid was extracted to obtain the recombinant plasmid pETDuet-1-plc-XynA.
[0030] Expression and preparation of xylanase: The plasmid pETDuet-1-plc-XynA was transformed into E.coli BL21(DE3) host bacte...
Embodiment 3
[0034] Example 3: Co-expression of Glucose Isomerase and Phospholipase C in E. coli 3L Tank Fermentation
[0035] Construction of pETDuet-1-plc-glu recombinant plasmid: pETDuet-1-plc plasmid and pET24a(+)-glu of the glucose isomerase gene with NCBI number AAZ55638 cloned into pET24a(+) were subjected to Nde I and Xho I double-enzyme digestion, the digestion product was recovered by gel cutting, and then ligated with T4 ligase at 16°C overnight. The ligation product was transformed into E.coli JM109 competent cells, and the transformation product was coated with ampicillin content of 100 μg·mL -1 The LB agar plates were cultured at 37 °C overnight, and the transformants were selected with an ampicillin content of 100 μg·mL. -1 The LB liquid medium was cultured, and then the plasmid was extracted to obtain the recombinant plasmid pETDuet-1-plc-glu.
[0036] Expression and preparation of glucose isomerase: pETDuet-1-plc-glu was transformed into E.coli BL21(DE3) host bacteria, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com