Podophyllotoxin derivatives as well as synthetic method and application thereof
A technology of podophyllotoxin and derivatives, applied in the field of preparation and its application in tumor suppression, which can solve the problems of poor water solubility and narrow anticancer spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0016] Example 1: Preparation of podophyllotoxin derivatives
[0017] At room temperature, add podophyllotoxin, corresponding carboxylic acid, refined dichloromethane and catalyst to a 50mL round-bottomed flask in sequence. TLC detects that the reaction is complete, and the corresponding podophyllotoxin derivatives are obtained by column chromatography. The physicochemical data of the corresponding compounds are as follows:
[0018] Compound 1: Yield: 77.3%, Mp: 116~118℃. 1 H NMR (300MHz, CDCl 3 )δ7.88-7.69 (m, 4H), 7.29-7.11 (m, 5H), 6.82-6.69 (m, 1H), 6.54 (d, J=8.2Hz, 1H), 6.42-6.25 (m, 2H) , 5.99(d, J=5.8Hz, 2H), 5.93(s, 1H), 5.31(dd, J=8.9, 4.8Hz, 1H), 4.61(s, 1H), 4.59-4.48(m, 1H), 4.27(d, J=7.8Hz, 1H), 3.88-3.67(m, 9H), 3.56-3.39(m, 2H), 2.97-2.92(m, 1H), 2.19(s, 1H).
[0019] Compound 2: Yield: 73.8%, Mp: 119~121℃. 1 H NMR (300MHz, CDCl 3 )δ7.82-7.67 (m, 4H), 7.25-7.06 (m, 5H), 6.78-6.66 (m, 1H), 6.49 (d, J=8.9Hz, 1H), 6.39-6.21 (m, 2H) , 5.95(d, J=5.7Hz, 2H), 5...
example 2
[0036] Example 2: Application of Formula I Class Podophyllotoxin Derivatives
[0037] We studied the anti-tumor activity of podophyllotoxin derivatives of formula I, selected tumor cells MCF-7, A549, and Hela as detection cells, and used MTT colorimetry as the detection method, and measured their absorbance at 570nm with a microplate reader And calculate the OD value.
[0038] IC 50 The value calculation method is as shown in the formula:
[0039] Cell inhibition rate (%)=(OD value of control group-OD value of experimental group) / OD value of control group×100%
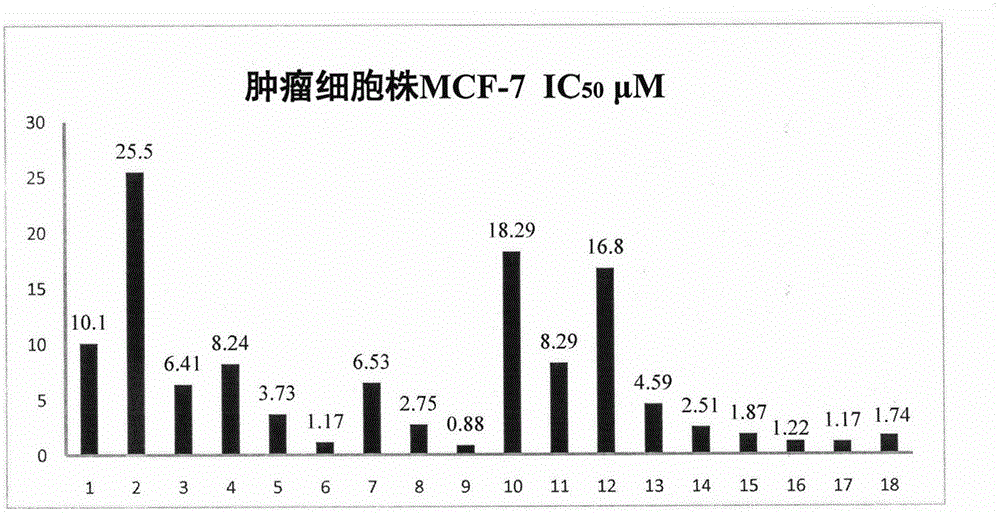
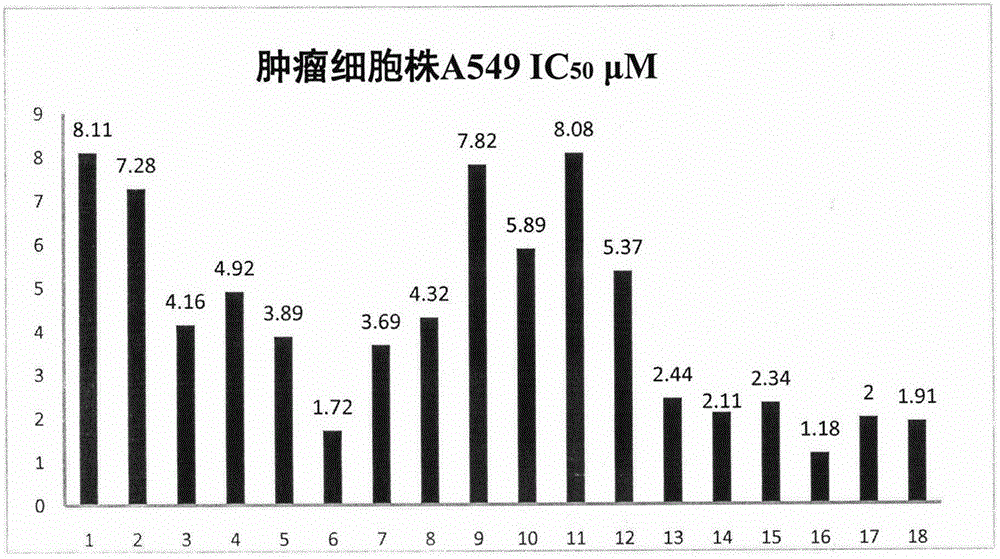
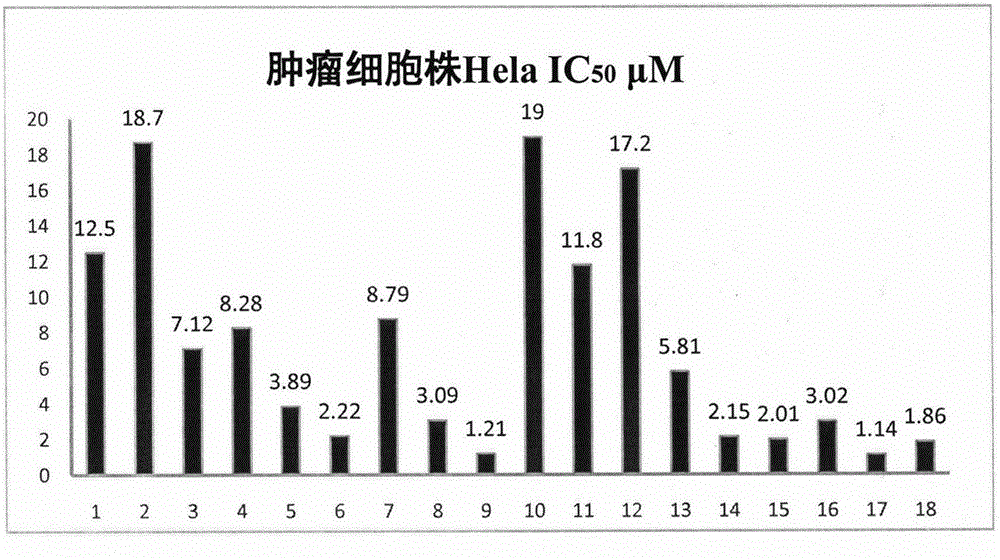
[0040] The results show that podophyllotoxin derivatives have certain potential in anti-tumor. The corresponding results are attached figure 1 , 2 , 3.
[0041] The results of cell activity showed that the activity of compound 9 was significantly better than other derivatives, and the IC of MCF-7, A549 and Hela cell lines were respectively 50 The values were 0.88 μM, 7.82 μM, and 1.21 μM.
example 4
[0042] Example 4: Compound 9 blocks cell cycle in G2 / M phase
[0043]MCF-7 cells were treated with compounds at different concentrations (0, 1, 2, 4 μM) for 24 hours, and the cells were collected for detection by flow cytometry. The results are shown in the appendix Figure 4 .
[0044] The podophyllotoxin derivatives of the present invention can be prepared into antitumor drugs.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com