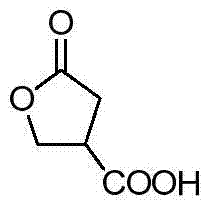
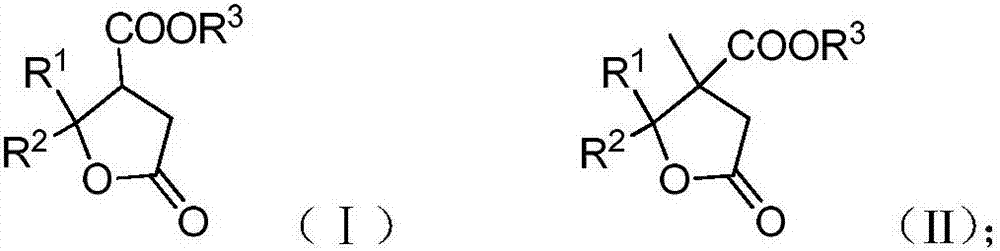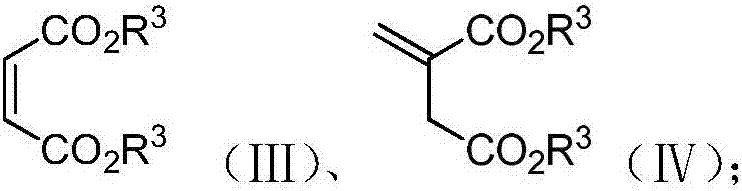A kind of enantioselective synthesis method of β-ester group-γ-butyrolactone
An enantioselective, butyrolactone technology, applied in the chemical industry, can solve the problems of no enantioselectivity or limitations of the synthesis process, and achieve the effects of high yield, high selectivity and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Under nitrogen atmosphere, add CuF(PPh 3 ) 3 · 2MeOH (17.3 mg, 0.0185 mmol), (S)-BINAP (17.5 mg, 0.0281 mmol), toluene (6 mL), stirred at 20° C. for 30 min. Join (CH 3 ) 3 Si(OSiHMe) r OSi(CH 3 ) 3 (PMHS) (mean value of r = 30, 0.10 mL, 1.67 mmol), stirred for 30 min. A mixture of acetophenone (0.070 mL, 0.60 mmol) and dimethyl maleate (0.10 mL, 0.78 mmol) (1 mL of toluene) was added. After reacting for 1h, add 5mL of 1.5M NH 4 F solution (5 mL), stirred for 0.5 h. Remove the insoluble matter by filtration with diatomaceous earth, wash the filter cake with dichloromethane, separate the phases of the filtrate, extract the aqueous phase with dichloromethane (3×5mL), wash the combined organic phase with saturated brine, and wash the organic phase with Dried over anhydrous sodium sulfate, concentrated by rotary evaporation, and separated by column to obtain β-methoxyl-γ-methyl-γ-phenyl-γ-butyrolactone (0.138g), with a yield of 98%. The content of each isomer was a...
Embodiment 2
[0052] Under nitrogen atmosphere, add CuF(PPh 3 ) 3 · 2MeOH (5.6 mg, 0.0060 mmol), (S)-BINAP (5.6 mg, 0.0060 mmol), toluene (10 mL), stirred at 20° C. for 30 min. PMHS (average value of r=30) (0.10 mL, 1.67 mmol) was added and stirred at 0° C. for 40 min. A mixture of acetophenone (0.070 mL, 0.60 mmol) and dimethyl maleate (0.10 mL, 0.78 mmol) (1 mL of toluene) was added. After reacting for 1h, add 5mL of 1.5M NH 4 F solution (5 mL), stirred for 0.5 h. Remove the insoluble matter by filtration with diatomaceous earth, wash the filter cake with dichloromethane, separate the phases of the filtrate, extract the aqueous phase with dichloromethane (3×5mL), wash the combined organic phase with saturated brine, and wash the organic phase with Dried over anhydrous sodium sulfate, concentrated by rotary evaporation, and separated by column to obtain β-methoxyl-γ-methyl-γ-phenyl-γ-butyrolactone (0.136g), with a yield of 97%. The content of each isomer was analyzed by chiral GC, ee ...
Embodiment 3
[0054] Under nitrogen atmosphere, add CuF(PPh 3 ) 3 · 2MeOH (5.6 mg, 0.0060 mmol), (S)-BINAP (5.6 mg, 0.0060 mmol), THF (2 mL), stirred at 20° C. for 30 min. PMHS (average value of r=30) (0.10 mL, 1.67 mmol) was added and stirred at 40° C. for 40 min. The temperature was raised to 60° C., and a mixture (1 mL THF) of acetophenone (0.070 mL, 0.60 mmol) and dimethyl maleate (0.10 mL, 0.78 mmol) was added. React for 1h, add NH 4 F solution (5 mL), stirred for 0.5 h. Remove the insoluble matter by filtration with diatomaceous earth, wash the filter cake with dichloromethane, separate the phases of the filtrate, extract the aqueous phase with dichloromethane (3×5mL), wash the combined organic phase with saturated brine, and wash the organic phase with Dried over anhydrous sodium sulfate, concentrated by rotary evaporation, and separated by column to obtain β-methoxyl-γ-methyl-γ-phenyl-γ-butyrolactone (0.127g), with a yield of 91%. The content of each isomer was analyzed by chir...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com