Novel crystal form of ledipasvir and preparation method thereof
一种晶型、溶剂化物的技术,应用在药物化学领域,能够解决无定形、不能很好地满足实际应用需求等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0154] The present invention also provides a kind of preparation method of described solvate, described method comprises the steps:
[0155] 1) Provide ledipasvir amorphous powder, the mixed solvent of component A and component B;
[0156] 2) mixing the ledipasvir amorphous powder and the mixed solvent, and crystallizing to obtain the solvate.
[0157] In another preferred example, the HPLC purity of the amorphous powder of ledipasvir is 85-98%, preferably 90-98%, more preferably 95-98%.
[0158] In the present invention, the volume ratio of component A and component B in the mixed solvent is not particularly limited, and can be adjusted in a wide range according to actual needs.
[0159] Typically, the volume ratio of component A to component B in the mixed solvent is 1:1-5, preferably 1:1-4, more preferably 1:1-3.
[0160] In the present invention, the addition ratio of the ledipasvir amorphous powder and the mixed solvent is not particularly limited, and can be adjusted i...
Embodiment 1
[0212] Embodiment 1 Preparation of ledipasvir crystal form A
[0213] Weigh 100 mg of ledipasvir amorphous powder into a 1.5 ml centrifuge tube, add 0.25 ml of acetonitrile (ACN) and methyl tert-butyl ether (MTBE) mixed solvent (volume ratio 1:3), and sonicate until dissolved. Place this solution at room temperature under airtight condition. After 3 days, a solid precipitate was observed, which was confirmed to be crystalline by polarizing microscopy.
[0214] Place the centrifuge tube in a centrifuge (Eppendorf mini spin) at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour to obtain Form A (LDV-acetonitrile-MTBE ternary solvates).
[0215] result
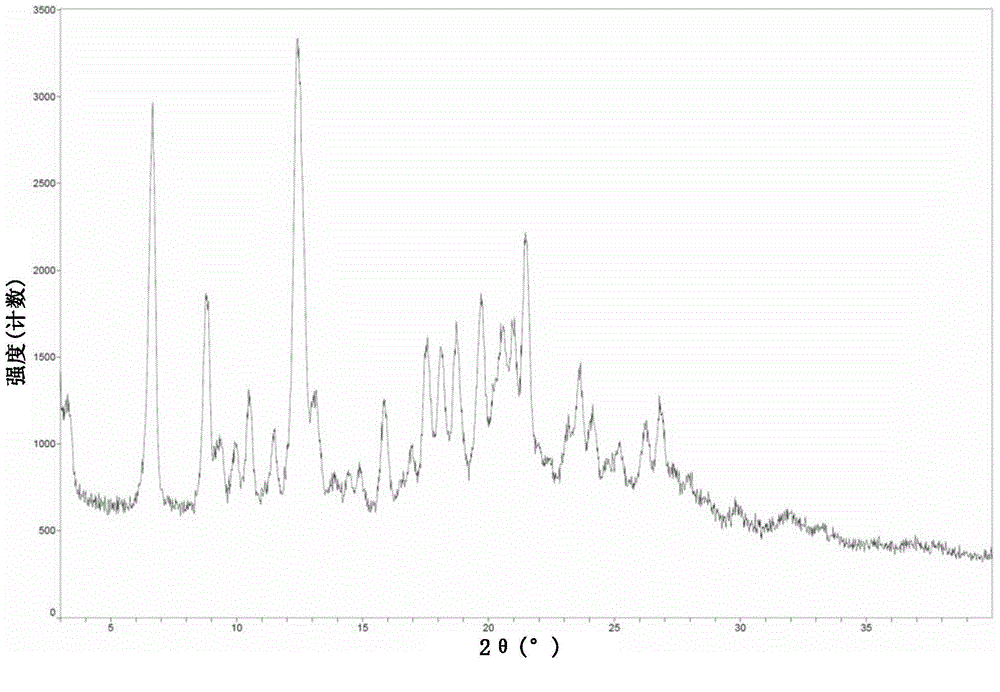
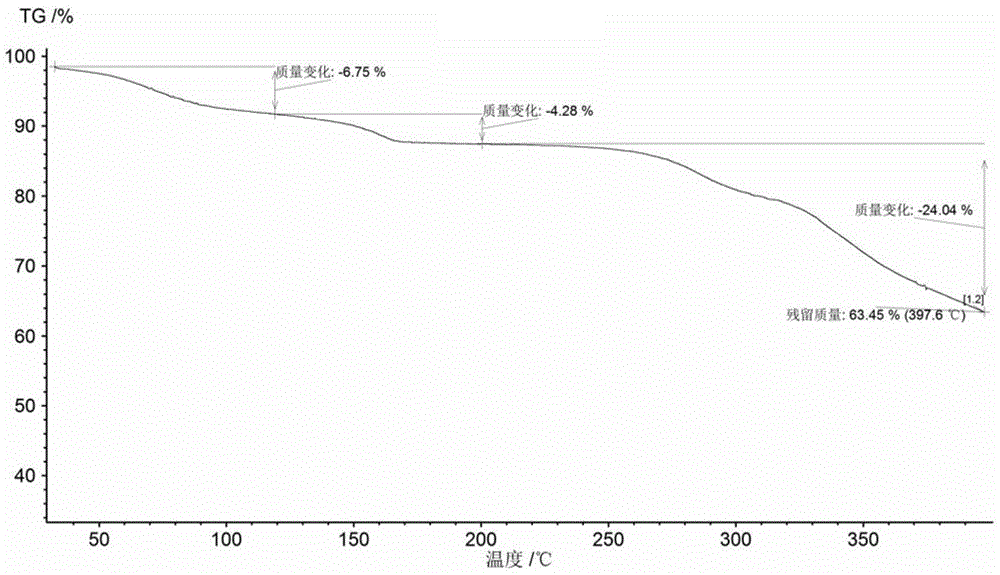
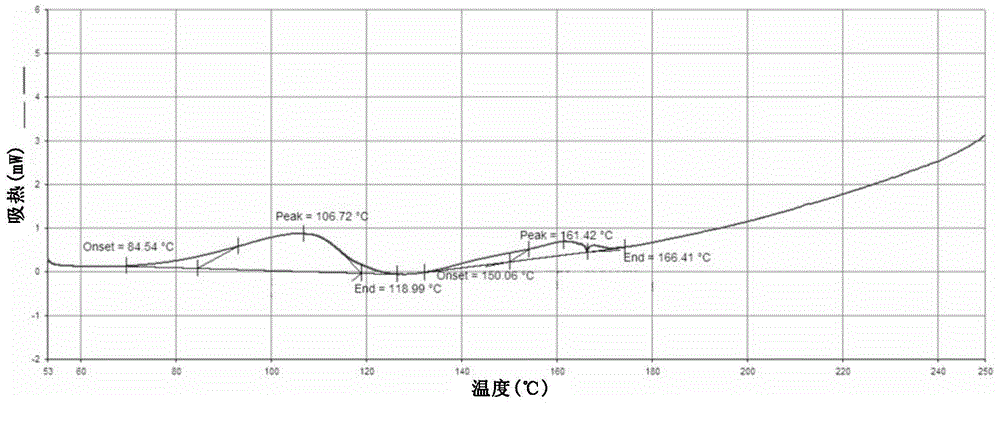
[0216] The product obtained in Example 1 was detected by XRD, DSC and TGA.
[0217] figure 1 It is the XRD figure of embodiment 1 crystal form A of the present invention. from figure 1 It can be seen that the main diffraction peaks and relative intensities of crysta...
Embodiment 2
[0225] Embodiment 2 Preparation of ledipasvir crystal form B
[0226]Weigh 40 mg of ledipasvir amorphous powder into a 1.5 ml centrifuge tube, add 0.2 ml of a mixed solvent of acetone (Acetone) and MTBE (volume ratio 1:2) to form a suspension. The suspension was left at room temperature and shaken for 3 days. After 3 days, a solid precipitate was observed, which was confirmed to be crystalline by polarizing microscopy.
[0227] Place the centrifuge tube in a centrifuge at 12,000 rpm for 5 minutes, remove the supernatant, and dry the separated solid at room temperature for 1 hour to obtain Form B (LDV-acetone-MTBE ternary solvate).
[0228] result
[0229] Figure 4 It is the XRD figure of embodiment 2 of the present invention Form B. from Figure 4 It can be seen that the main diffraction peaks and relative intensities of the crystal form B obtained in Example 2 are shown in Table 2.
[0230] Table 2. XRD data of ledipasvir crystal form B
[0231] 2θ position [...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com