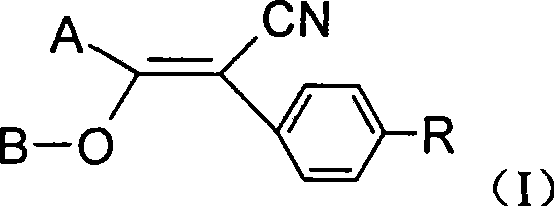
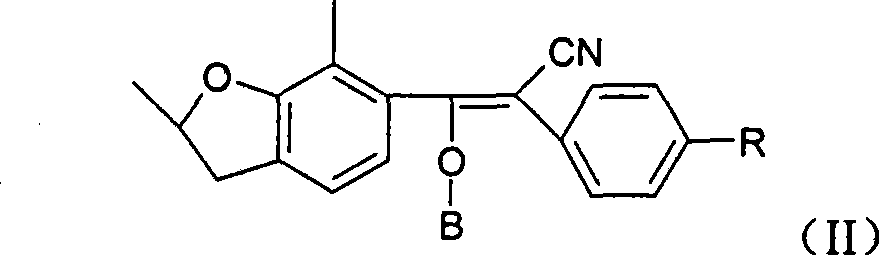
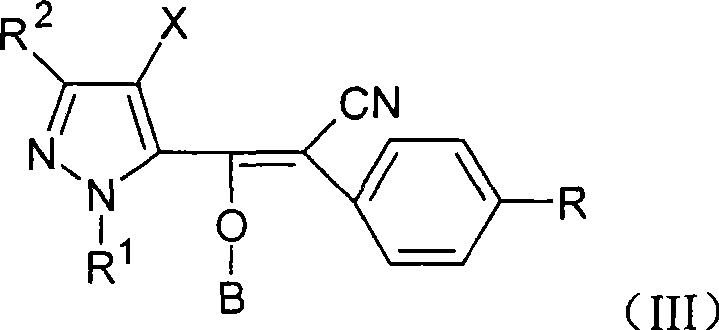
Vinyl cyanide compounds, preparation and application thereof
A technology of acrylonitrile and compounds, applied in the field of pesticides, can solve the problems of ecosystem damage, high toxicity of pesticides, residues, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Example 1: Synthesis of 2-(4-tert-butylphenyl)-3-(2,3-dihydro-2,7-dimethyl-6-benzofuryl)-3-hydroxyacrylonitrile
[0058] At 50°C, a solution formed by dissolving 5.0g of 4-tert-butylphenylacetonitrile in 15ml of THF was added dropwise to a suspension formed by 3.0g of 50% sodium hydride in 50ml of THF, and the resulting product was stirred for 30 minutes, then At 50°C, a solution of 6.5 g of 2,3-dihydro-2,7-dimethyl-6-benzofuranoyl chloride dissolved in 15 ml of THF was added dropwise, and then stirred overnight at room temperature. The reaction mixture was desolvated under reduced pressure and poured into water, added 5.0 g of hydrochloric acid and stirred for 30 minutes, extracted with 100 ml of chloroform, the organic layer was washed with saturated brine and dried with anhydrous sodium sulfate, and desolvated under reduced pressure to obtain 6.5 g of brown viscous things.
Embodiment 2
[0059] Example 2: 2-(4-tert-butylphenyl)-3-(2,3-dihydro-2,7-dimethyl-6-benzofuryl)-3-isobutyryloxypropene Nitrile synthesis
[0060] A solution consisting of 0.5g of isobutyryl chloride and 5ml of chloroform was slowly added dropwise under stirring and cooling in an ice-water bath to 1.0g of 2-(4-tert-butylphenyl)-3-(2,3-dihydro-2, 7-dimethyl-6-benzofuryl)-3-hydroxyacrylonitrile, 0.6g triethylamine and 20ml chloroform in the mixed solution. After the dropwise addition, the temperature of the reaction mixture was raised to about 30°C, and the stirring was continued at this temperature, and the chromatographic tracking was carried out until the reactant was basically converted completely, which took about 5-6 hours. The resulting reaction mixture was poured into water (30 ml), and the layers were separated upon standing. The organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, and precipitated under reduced pressure. The residue was the title com...
Embodiment 3
[0062] According to the method described in Example 2, the corresponding acid chloride, sulfonyl chloride, phosphorus oxychloride and 2-(4-tert-butylphenyl)-3-(2,3-dihydro-2,7 -Dimethyl-6-benzofuryl)-3-hydroxyacrylonitrile Synthesis of other acrylonitrile compounds described in Table 1 were prepared. Such as reacting acetyl chloride with the compound obtained in Example 1 to prepare No.1 compound; reacting isovaleryl chloride with the compound obtained in Example 1 to prepare No.2 compound; reacting benzoyl chloride with the compound obtained in Example 1 to prepare No.3 compound (±) cis-trans-2,2-dimethyl-3-(2,2-dichlorovinyl) cyclopropanyl formyl chloride reacts with the compound obtained in Example 1 to prepare No.4 compound; Propionyl chloride and Example Compound No.5 was prepared by reacting the compound obtained in 1; Compound No.7 was prepared by reacting t-valeryl chloride with the compound obtained in Example 1; Compound No.8 was prepared by reacting methanesulfonyl ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com