Bleaching process and device used in nitric acid preparation process
A process, nitric acid technology, applied in the field of bleaching process and its devices, can solve the problems of taking a long time, consuming a lot of electric energy or heat energy, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
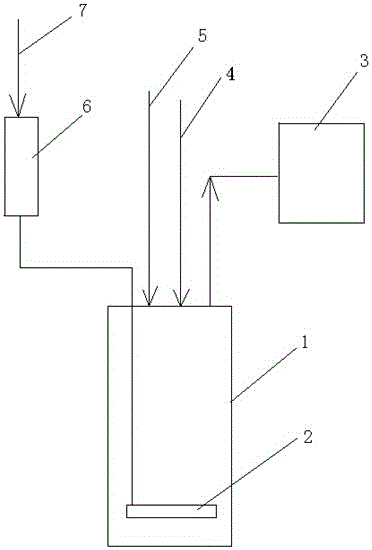
[0023] Such as figure 1 Shown, a kind of device of the bleaching process in the preparation process of nitric acid comprises acid distribution tank 1, and the top outlet of described distribution acid tank 1 is connected with negative pressure pump 3, and the inlet end of distribution acid tank 1 is connected with hydrogen peroxide storage tank 6. The outlet end is connected, the top inlet of the hydrogen peroxide storage tank 6 is connected to the compressed air pipe 7, and a gas distributor 2 is horizontally arranged inside the acid distribution tank 1 .
[0024] That is, the hydrogen peroxide in the hydrogen peroxide storage tank 6 is driven by the compressed air in the compressed air pipe 7, and evenly enters the acid liquid in the acid distribution tank 1 through the gas distributor 2 to react with the nitrogen dioxide therein. The oxygen produced at the same time drives excess nitrogen dioxide to escape from the nitric acid solution, which is extracted and charged by neg...
Embodiment 2
[0027] A kind of bleaching process in the preparation process of nitric acid, comprises the following steps:
[0028] (1) Pump the acid tank for preparing nitric acid to a slight negative pressure of -10Pa;
[0029] (2) With compressed air as the power, hydrogen peroxide is introduced into the nitric acid solution in the acid distribution tank, and the hydrogen peroxide enters the nitric acid solution evenly through the gas distributor and stirred evenly, so that the hydrogen peroxide and the NO in the acid distribution tank 2 The molar ratio is 1.5:2;
[0030] (3) Recover excess NO at the outlet of the acid distribution tank 2 .
Embodiment 3
[0032] A kind of bleaching process in the preparation process of nitric acid, comprises the following steps:
[0033] (1) Pump the acid tank for preparing nitric acid to a slight negative pressure of -5Pa;
[0034] (2) With compressed air as the power, hydrogen peroxide is introduced into the nitric acid solution in the acid distribution tank, and the hydrogen peroxide enters the nitric acid solution evenly through the gas distributor and stirred evenly, so that the hydrogen peroxide and the NO in the acid distribution tank 2 The molar ratio is 1.2:2;
[0035] (3) Recover excess NO at the outlet of the acid distribution tank 2 .
[0036] The present invention uses hydrogen peroxide to evenly enter the solution in the acid distribution tank through a gas distributor (20 cm away from the bottom of the acid distribution tank), and the gas distributor evenly distributes to drive the NO in nitric acid 2 Reaction, conversion into nitric acid, and at the same time, the nitrogen di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com