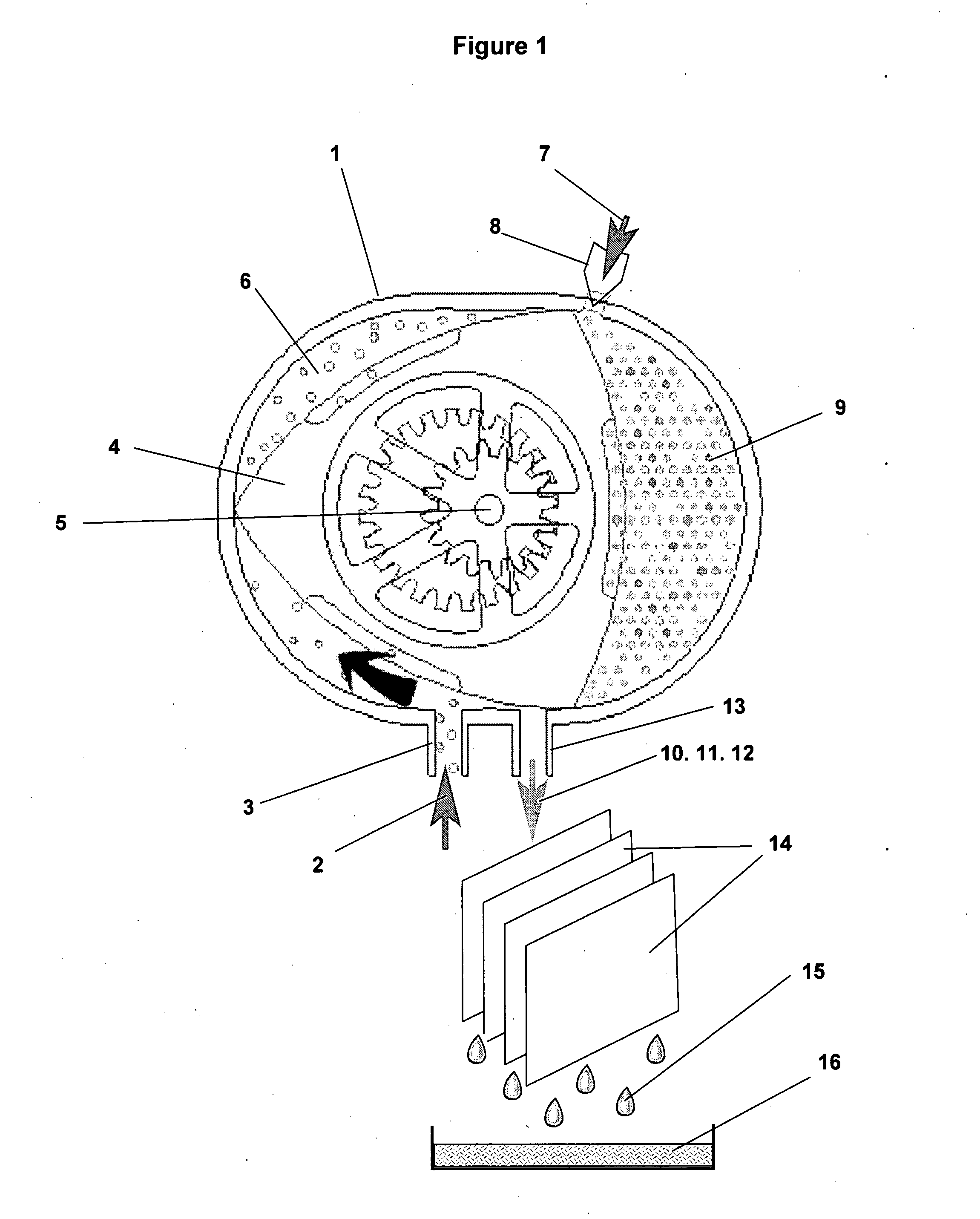
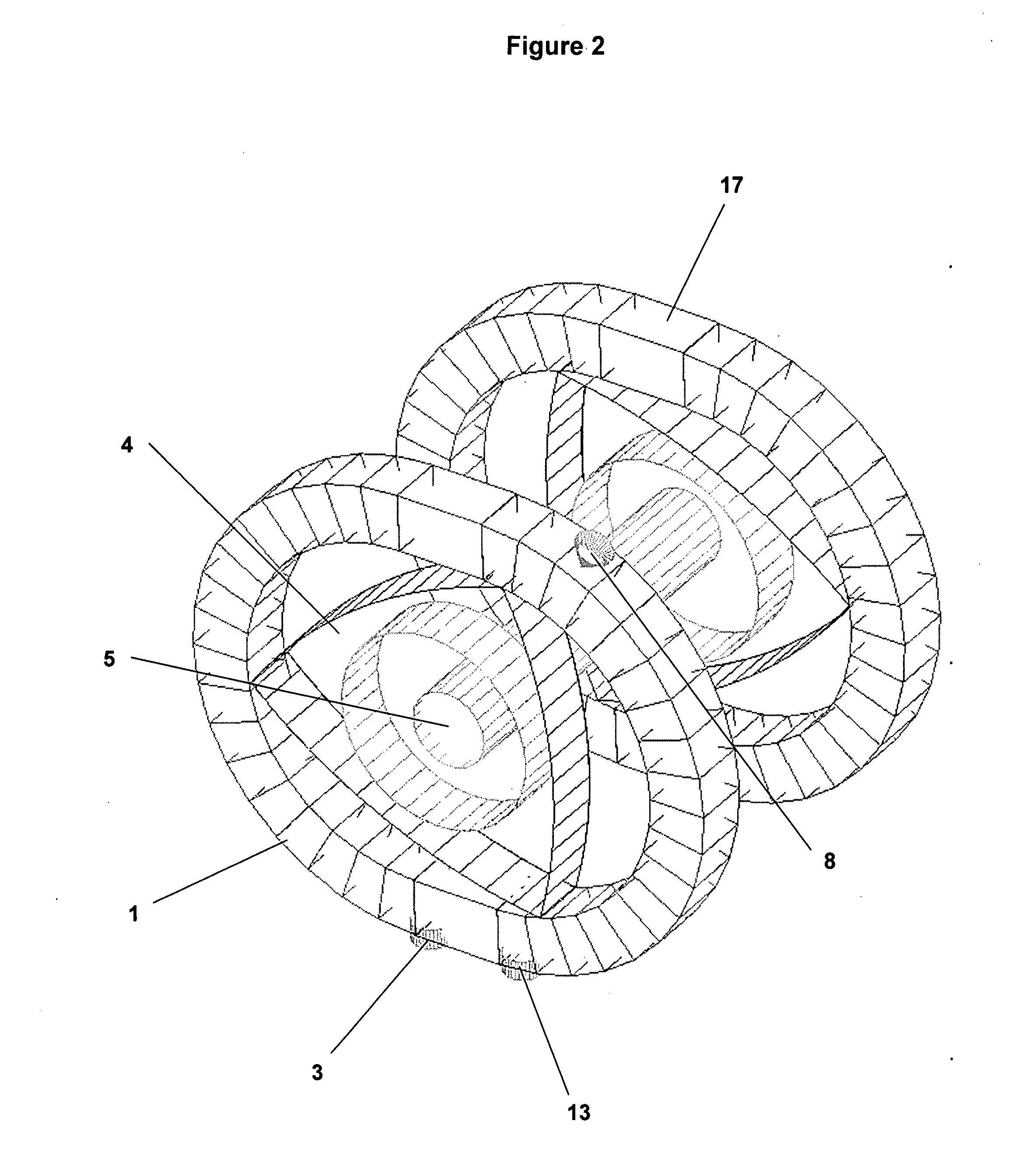
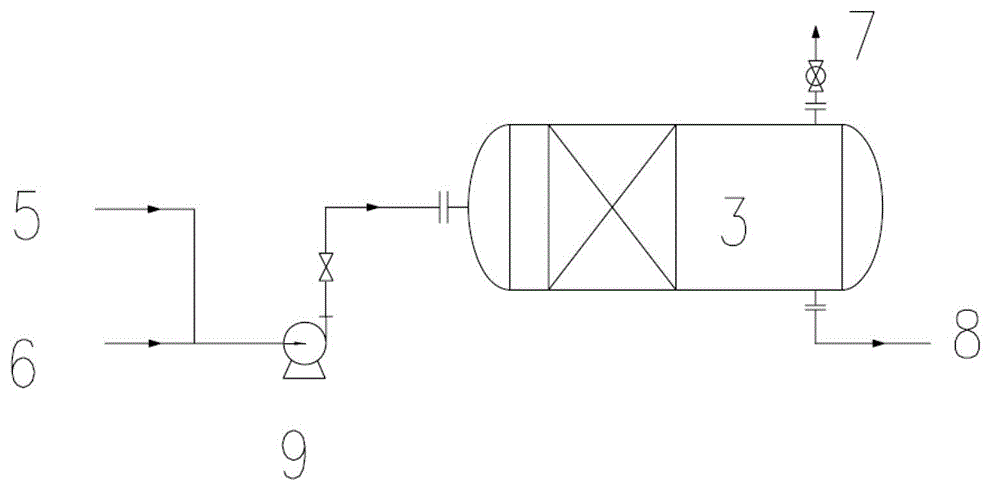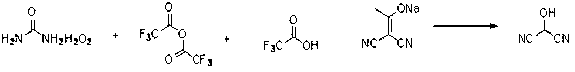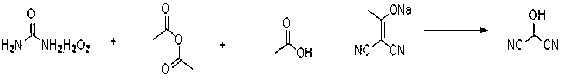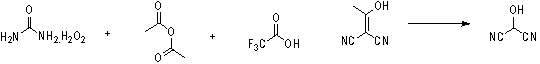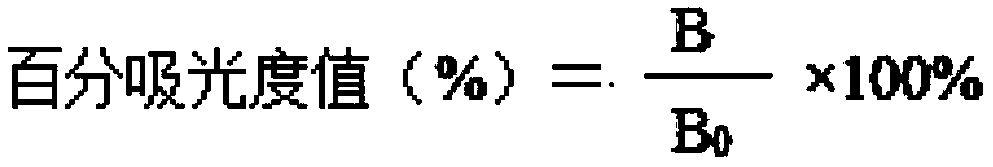Patents
Literature
84 results about "Urea hydrogen peroxide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
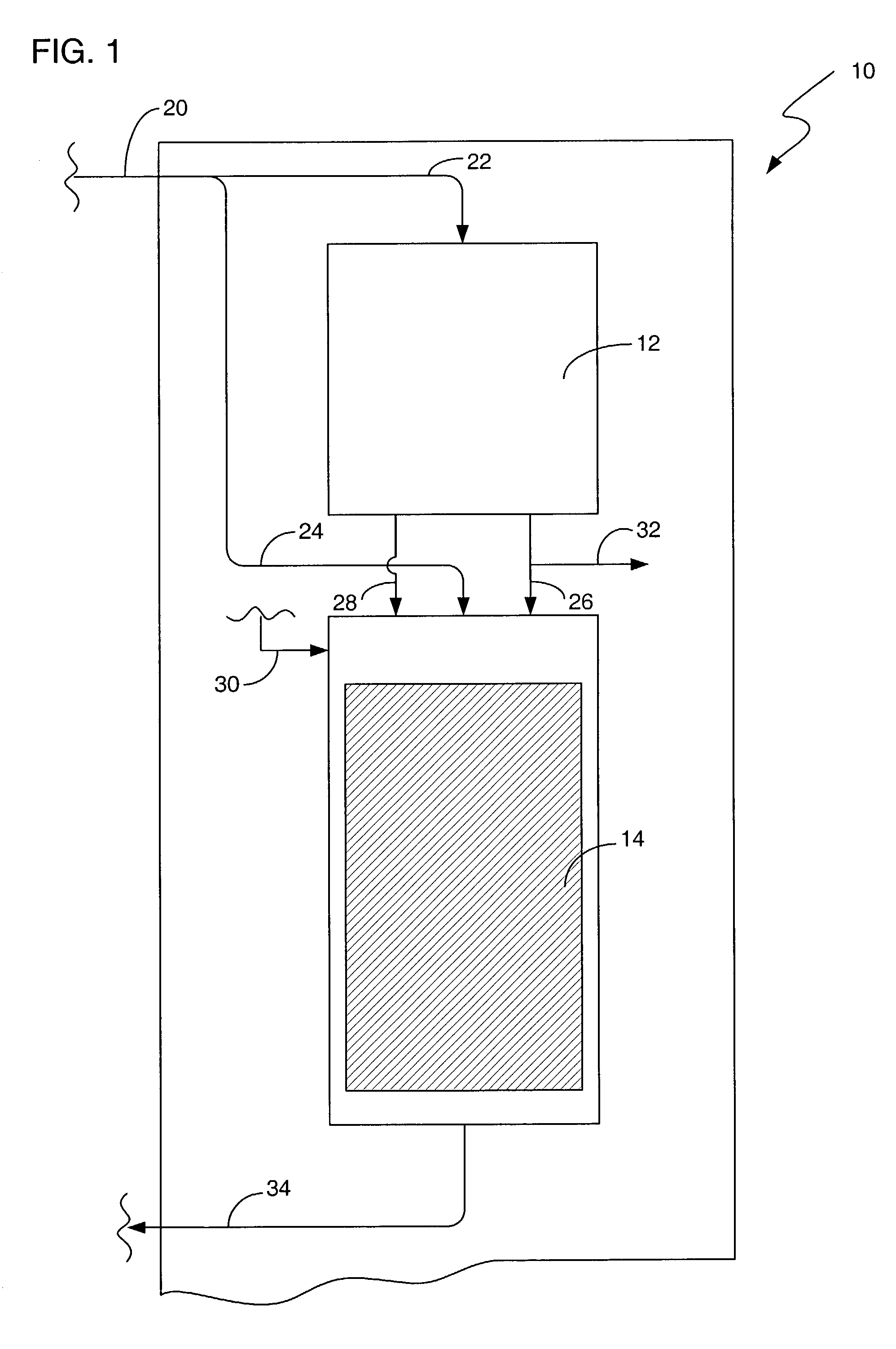
Hydrogen peroxide - urea (also called Hyperol, artizone, urea hydrogen peroxide, and UHP) is a solid composed of equal amounts of hydrogen peroxide and urea. This compound is a white crystalline solid which dissolves in water to give free hydrogen peroxide.
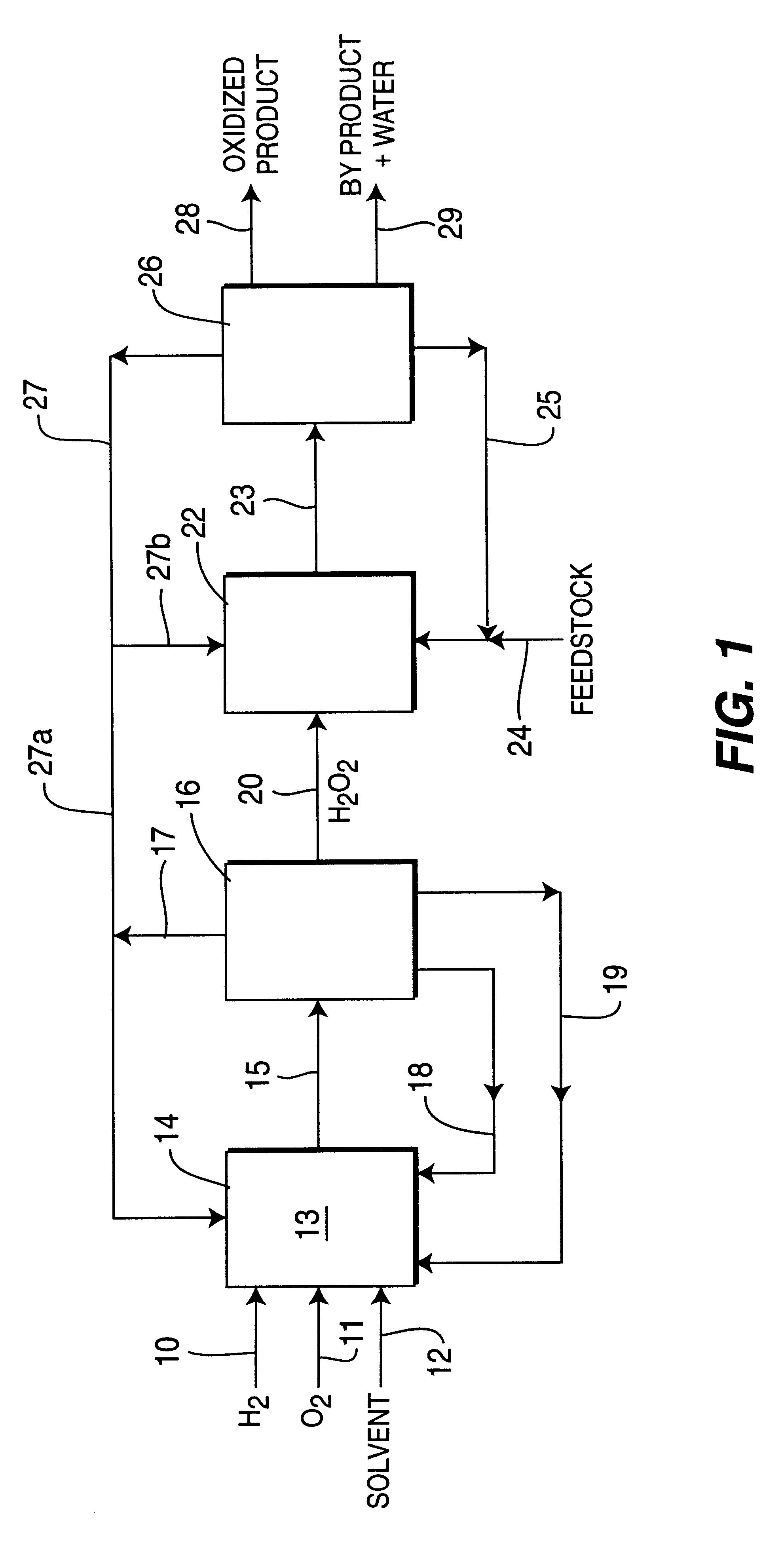
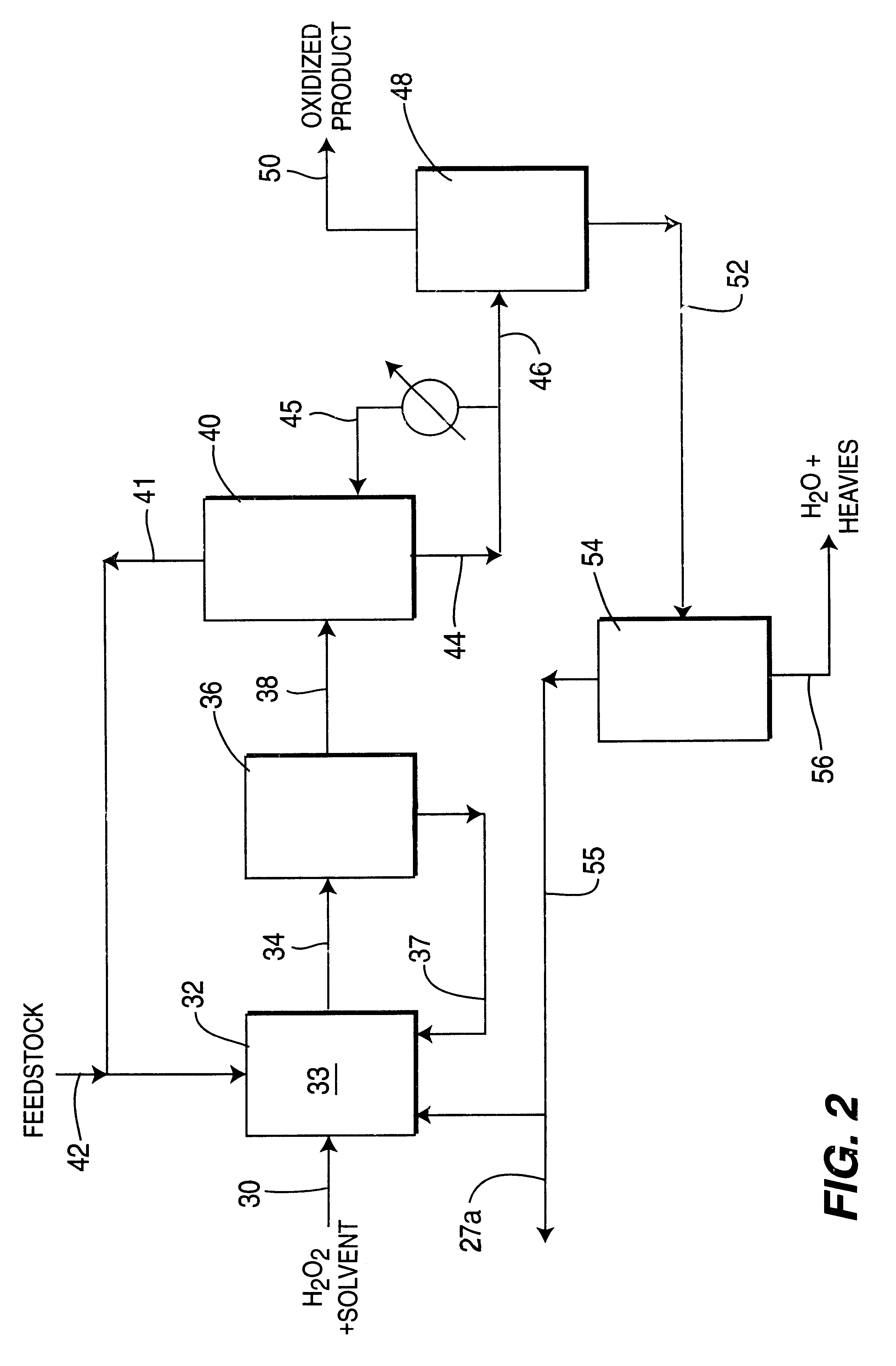
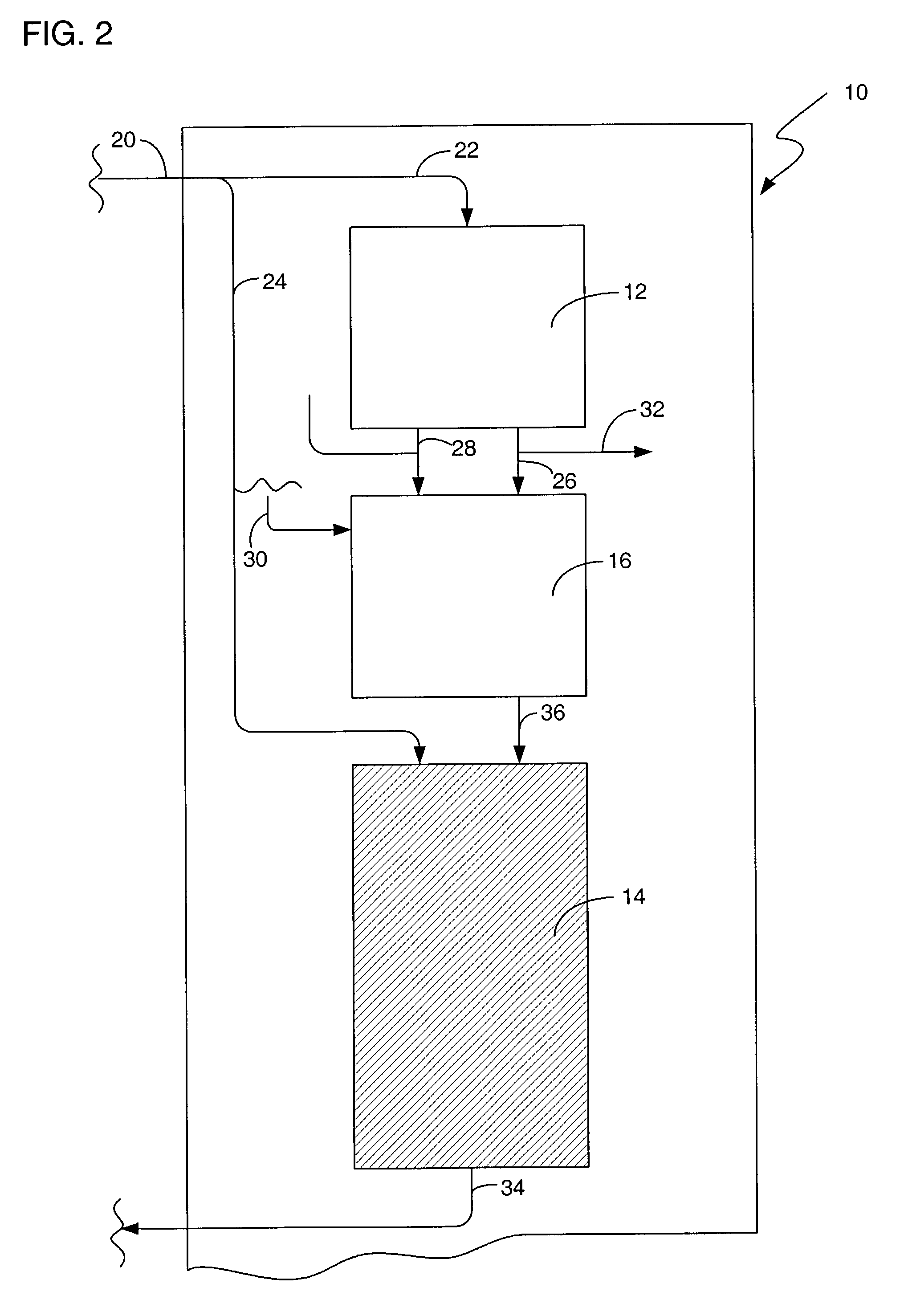
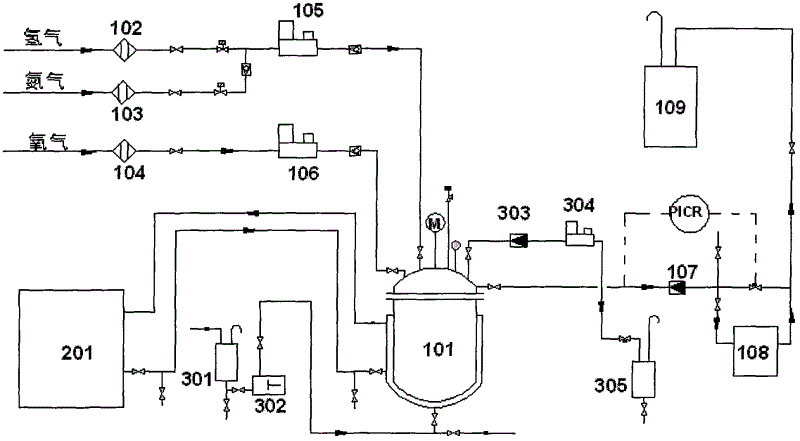
Process for selective oxidation of organic feedstocks with hydrogen peroxide
InactiveUS6500968B2Increase productionLow costOrganic chemistryPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesDistillationActive support
A process for producing oxidized organic chemical products such as propylene oxide from various organic chemical feedstocks utilizing as oxidant directly produced hydrogen peroxide (H2O2) intermediate oxidizing agent. The hydrogen peroxide intermediate is directly produced from hydrogen and oxygen feeds plus a suitable solvent in a first catalytic reaction step utilizing an active supported phase-controlled noble metal catalyst at reaction conditions of 0-100° C. temperature and 300-3,000 psig pressure. An organic chemical feedstock such as propylene together with the hydrogen peroxide intermediate and solvent solution are fed into a second catalytic reactor maintained at 0-150° C. temperature and 15-1,500 psig pressure and oxidized to produce a desired crude oxidized organic product such as propylene oxide, which is purified by distillation steps and recovered from the solvent solution.
Owner:HEADWATERS TECH INNOVATION LLC
Hydrogen peroxide working solution and its application in preparation of hydrogen peroxide
ActiveCN103588177AReduce solubilityImprove solubilityPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesOrganic solventPhosphate
The invention belongs to the technical field of hydrogen peroxide, and especially relates to a hydrogen peroxide working solution and its application in the preparation of hydrogen peroxide. The hydrogen peroxide working solution comprises 2-ethylanthraquinone having a mass concentration of 120-200g / L and an organic solvent, and the organic solvent comprises, by volume, 70-80% of a C9-C10 aromatic hydrocarbon, 14-20% of trioctyl phosphate, 4-10% of 2-methylcyclohexyl acetate and 0.4-0.9% of tertiary amine When the hydrogen peroxide working solution is used in the preparation of hydrogen peroxide, the hydrogen peroxide working solution can greatly improve the hydrogenation efficiency and the oxidation efficiency of 2-ethylanthraquinone, and has the advantages of low production cost, no peculiar smell and product yield improvement.
Owner:WEIFANG MENJIE CHEM
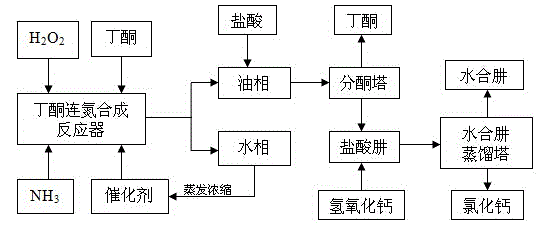
Preparation method of hydrazine hydrate
The invention belongs to the technical field of chemical synthesis, particularly relates to a preparation method of hydrazine hydrate and aims at improving production process of a hydrogen peroxide method and provides a hydrazine hydrate preparation method which is low in energy consumption, high in yield and free of pollution. The hydrazine hydrate preparation method provided by the invention comprises the following steps: carrying out reaction on ammonia, hydrogen peroxide and butanone in the presence of a catalyst to generate methyl ethyl azine; separating the methyl ethyl azine -containing upper oil phase from the catalyst-containing water phase; concentrating the catalyst-containing water phase to reuse; acid decomposing the methyl ethyl azine into hydrazonium salt and butanone, recycling the butanone, neutralizing the hydrazonium salt into hydrazine hydrate by alkali and distilling and recovering to obtain a hydrazine hydrate solution. The method can be used for preparing hydrazine hydrate and is high in yield and free of pollution.
Owner:重庆锦杉科技有限公司
Gentle preservative compositions
InactiveUS20050266095A1Improve anti-corrosion performanceIncreasing lens wearer comfortBiocideOrganic detergent compounding agentsNatural productPreservative
Compositions are described which are useful in preserving any topically applied solution. Compositions including urea-hydrogen peroxide complex in solution in an amount effective to preserve contact lenses are also described, as well as methods of making and using such solutions. A hydrogen peroxide component of such solutions, when introduced into the eye, is converted to oxygen and water, leaving only the natural product, urea.
Owner:BAUSCH & LOMB INC
Preparation method of starch base paper packing adhesive
InactiveCN101838508AEasy to transportEasy to useNon-macromolecular adhesive additivesStarch derivtive adhesivesAdhesiveOxidizing agent
The invention belongs to the technical field of the adhesive, and particularly relates to a preparation method of starch base paper packing adhesive, which comprises the steps of mixing, oxidizing and modifying, wherein the mixing step is: water, Urea hydrogen peroxide and starch are added into a reactor to be uniformly mixed, and the mass ratio of the water, the Urea hydrogen peroxide and the starch is: 10 to 16 parts of water, 1 to 4 parts of Urea hydrogen peroxide and 8 to 12 parts of starch; the oxidizing step is: the pH value is adjusted to 8 to 10, catalyst is added to be mixed for 20 to 40 minutes, and then the mixture is statically held for1 to 2 hours; and the modifying step is: modifier and filler are added to be uniformly mixed. The Urea hydrogen peroxide is used as the oxidant of the starch, so not only the production cost is low, the glue consumption quantity is less, but also the production process is free from utilizing defoamer and is free from peculiar smell, and the environmental protection can be realized.
Owner:HUZHOU SIYI STARCH IND
Apparatus and process for the synthesis of hydrogen peroxide directly from hydrogen and oxygen
InactiveUS7115192B1Raise the ratioIncrease concentrationProcess control/regulationHydrogen peroxideHydrogenElectrolysis of water
Owner:UOP LLC
Method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide
ActiveCN104610071AThe concentration of hydrochloric acid does not changeThe reaction process is stable and easy to controlOrganic compound preparationAmino compound preparationP-NitroanilineReaction system
The invention relates to a method for preparing 2,6-dichloro-4-nitroaniline through direct chlorination of chlorine gas and oxidative chlorination of hydrogen peroxide. The method comprises the following steps: (1) adding paranitroaniline into hydrochloric acid at the concentration of 5-35wt%, heating to 40-80 DEG C, stirring for uniformly mixing, slowly inflating the chlorine gas within 0.5-5h, slowly adding hydrogen peroxide dropwise, and after adding the chlorine gas and the hydrogen peroxide, continuing to preserve heat and react for 0.3-1.5h; (2) filtering a product obtained in the step (1), washing a filter cake to be neutral, and drying to obtain the 2,6-dichloro-4-nitroaniline. By regulating a dosage ratio of the chlorine gas to the hydrogen peroxide, the concentration of the hydrochloric acid in a reaction system can be kept unchanged, the reaction process is steady and easily controlled, and the product is high in yield and purity.
Owner:昌邑新澳化工有限公司
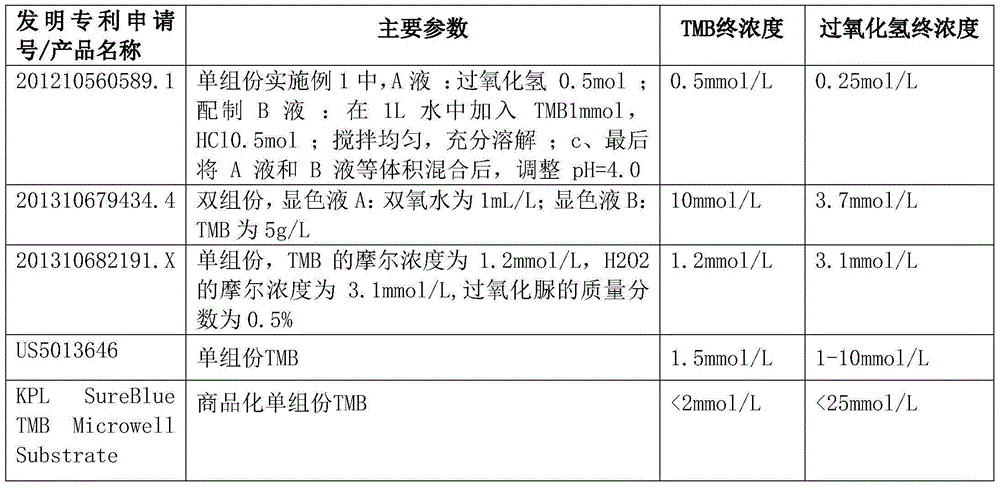
Single-component TMB coloration liquid and preparation method thereof
ActiveCN105116141AImprove solubilityGuaranteed stabilityMaterial analysisOrganic solventCarbamide peroxide
The present invention provides a single-component TMB coloration liquid, which is formed by mixing a liquid A and a liquid B, wherein urea hydrogen peroxide is dissolved in a citric acid-sodium citrate buffer liquid to prepare the liquid A, and polyvinyl alcohol, polyvinyl pyrrolidone, TMB and glycerol are dissolved in a citric acid-sodium citrate buffer liquid to prepare the liquid B. Compared with the single-component TMB coloration liquid in the prior art, the single-component TMB coloration liquid of the present invention has the following advantages that: the stability of the coloration substrate in the solution can be maintained, the operator can be protect from being away from organic solvent hazard, the damage on the environment can be reduced, the stable aqueous solution system can be formed, and the single-component TMB coloration liquid can be stored for 24 months at a temperature of 2-8 DEG C.
Owner:SHENZHEN WEIGUANG BIOLOGICAL PROD
Tooth powder and fabrication process thereof
InactiveCN102670457AImprove solubilityStrong complexing abilityCosmetic preparationsToilet preparationsIrritationPhosphate
The invention discloses a tooth powder which is characterized by comprising anti-staining active ingredient, acid-free desensitization ingredient, odour removal ingredient and base stock ingredient, wherein the anti-staining active ingredient comprises modified phytic acid sodium and urea hydrogen peroxide; the acid-free desensitization ingredient comprises sodium monofluorophosphate and Chinese medicine desensitization agent; the odour removal ingredient comprises angelica root extract and liex rotunda extract; and the base stock ingredient comprises dicalcium phosphate dehydrate, calcium carbonate, silica, soluble saccharin, glycerol, essence, sodium lauryl sulfate and the balance of preservative. When being in fabrication, the tooth powder not only has the functions of cleaning dental plaque, complexing and dissolving pigmentation of tooth surface but also can reduce the irritation to the oral cavity and the aching, limp and allergic feeding can not be caused during use process through a combination technology.
Owner:GUANGZHOU CHINA HAN ORAL PROD
Cold-humid adversity resisting tobacco coated seed and preparation method thereof
ActiveCN101828446AIncrease vitalityShort germination timeBiocidePlant growth regulatorsTemperature stressHigh resistance
The invention discloses a cold-humid adversity resisting tobacco coated seed and a preparation method thereof. Before being subjected to coating and pelleting, the tobacco seed is initiated by using mixed solution consisting of 5 to 10g / L urea hydrogen peroxide and 50 to 100mg / L gibberellin; and when coating and pelleting is performed, 10.0 to 20.0 percent calcium peroxide is added into a seed coating agent. The cold-humid adversity resisting tobacco coated seed prepared by the invention has the advantages of high seed activity, short sprouting time, high rate of emergence in the fame, uniform emergence of seedlings, high resistance to low temperature stress and hyproxia stress, and the like, and has excellent application prospect in the tobacco production.
Owner:YUNNAN ACAD OF TOBACCO AGRI SCI +1
Hydrogen peroxide-hydrochloric acid oxidation and desulfurization method
InactiveCN103184068AEasy to buyLow priceTreatment with plural serial refining stagesDispersityReaction temperature
The invention discloses a hydrogen peroxide-hydrochloric acid oxidation and desulfurization method. The hydrogen peroxide-hydrochloric acid oxidation desulfurization method comprises the following steps of: oxidizing sulfide in fuel oil into sulphone or sulfoxide with strong polarity in an acid medium by using hydrogen peroxide, and extracting the sulphone or sulfoxide by using a polar solvent, thus removing sulfide in the fuel oil; controlling the potential of an oxidizing agent and the reaction speed by controlling the concentration of the hydrogen peroxide and hydrochloric acid and reaction temperature; and reinforcing the dispersity of the fuel oil in water through an organic matter chaotropic agent, accelerating the reaction speed, and extracting the sulphone or sulfoxide from the fuel oil by using the polar solvent. The hydrogen peroxide-hydrochloric acid oxidation and desulfurization method has advantages of low cost, high oxidation and desulfurization efficiency and very good popularization and application prospect in petrochemical industries.
Owner:HUAIYIN TEACHERS COLLEGE
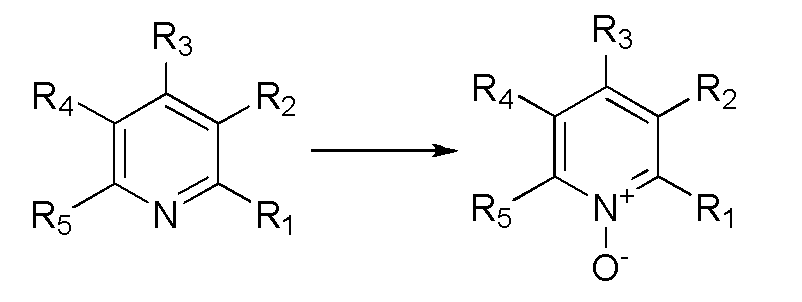
Synthetic method for preparing pyridine N-oxide
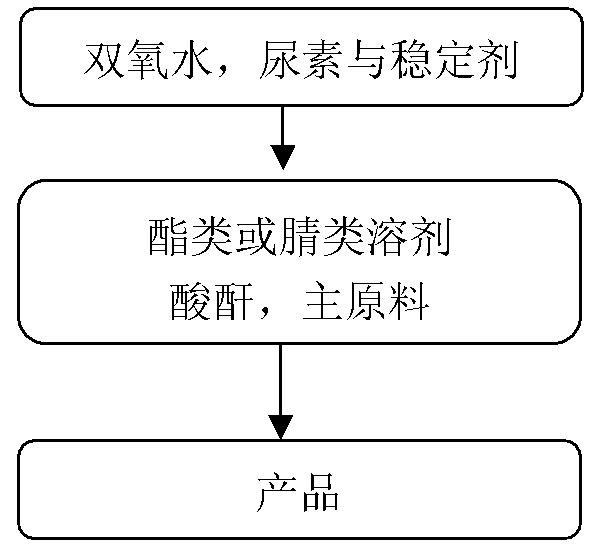
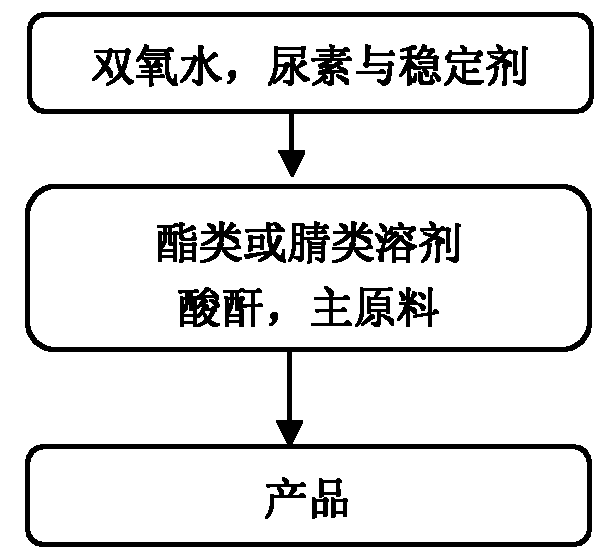
Provided is a synthetic method for preparing pyridine N-oxide. According to the method, raw materials which are commercialized on the market are selected, or pyridine, monosubstituted pyridine or polysubstituted pyridine derivatives which are easy to prepare are used as starting materials, and the materials are oxidized by urea-hydrogen peroxide compound so to prepare the final product. The advantages of the invention are as follows: 1, the raw materials used in the invention are easily available and cheap and are commercialized or easily preparable raw materials, meeting requirements for large scale production; 2, a safer oxidation method is employed in the invention, thereby avoiding condensation of acetate containing hydrogen peroxide and greatly improving safety factors of production; 3, chemical reaction conditions required in the invention are mild, the operation is simple, little pollution to environment is produced, and the method is technically mature for large scale production.
Owner:ASYMCHEM LAB TIANJIN
Methane conversion to methanol
InactiveUS20070270512A1Easy constructionEfficient productionCatalytic gas-gas reactionPreparation by oxidation reactionsWater vaporMethane gas
Disclosed are a method and apparatus with alternate embodiments that use commercial chemical free radical initiators to directly convert methane to methanol in a low energy, one step, catalytic reaction. In one embodiment an apparatus converts “packets” of methane gas to liquid methanol. Methane gas and aqueous hydrogen peroxide are injected into the packet apparatus through valved ports and mixed in a structurally robust, rotating reaction chamber coated with metal cation catalyst. Although operating at low temperature and pressure, the rotating chamber is sufficiently strong enough to contain spontaneous ignition of the mixture should that occur. The reactive oxygen species, primarily hydroxyl radicals, released in the chamber of mildly elevated temperature, oxidize the methane molecule, replacing one hydrogen atom on its molecule with one stable hydroxyl OH molecule, converting vapor methane CH4 to vapor methanol CH3OH. Vapor exiting the exhaust port condenses on ambient temperature drip screens to stable liquid methanol. An alternate continuous process embodiment uses flow-through conversion screens coated with immobilized dry chemicals. Water vapor entering the apparatus releases molecular oxygen from screens coated with dry urea hydrogen peroxide. A circulating endless belt screen is used to continual recoat the dry urea hydrogen peroxide. Reactive oxygen species are generated on successive conversion screens through which the molecular oxygen, and methane gas entering the apparatus, must pass. Metal cation catalyst molecules physically entrapped within interstitial cavities of a three dimensional, fixed porous colloidal matrix coating on the screens create powerful hydroxyl radicals from the molecular oxygen. These reactive species in turn convert methane molecules impacting the conversion screens to methanol molecules. The method and apparata operate at modest temperature, pressure, and energy requirement levels, and are scalable from portable units to industrial scale methanol refineries.
Owner:EDWARDS JOHN LEE
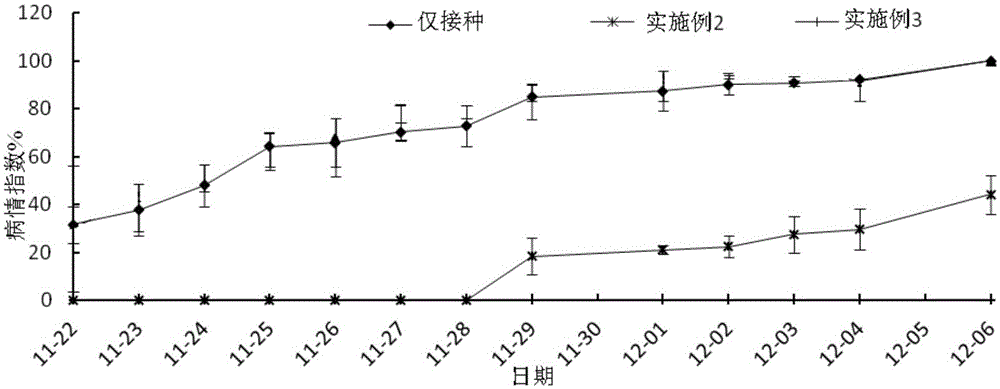
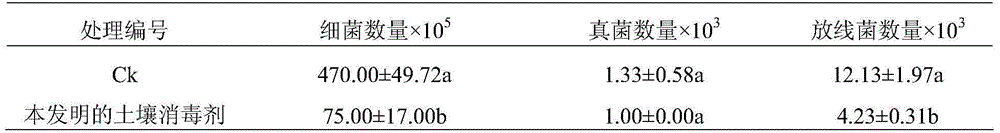
Soil disinfectant and method for preventing and treating phytophthora capsici
ActiveCN105104430AFree from the threat of soil-borne diseasesBiocidePlant growth regulatorsDisinfectantSlurry
The invention relates to soil disinfectant and a method for preventing and treating phytophthora capsici. A raw material of the soil disinfectant comprises the following components: urea-hydrogen peroxide and biogas slurry. The mass ratio of the urea-hydrogen peroxide to biogas slurry is (1-5): (95-99). By the soil disinfectant, the phytophthora capsici in soil can be killed, crops and seedlings can be prevented from being threatened by soil-borne diseases, certain nutrient substances are provided for plant growth, and residues are avoided.
Owner:INST OF AGRI RESOURCES & ENVIRONMENT SHANDONG ACADEMY OF AGRI SCI
Preparation of methyl ethyl ketazine by utilizing hydrogen peroxide method
InactiveCN103044285AChange the face of backwardnessSolve intractableHydrazone preparationHydrazine compoundKetone
The invention discloses a formula and a production process for preparing methyl ethyl ketazine by utilizing a hydrogen peroxide method, which carries out an in-depth study on the hydrogen peroxide method, thereby improving the hydrogen peroxide method to form a ketazine production method suitable for the situations in China. In the method, hydrogen peroxide is used as an oxidant, ammonia and butanone (methyl ethyl ketone) are used as raw materials and formamide and an inorganic oxide are used as a catalyst and a cocatalyst respectively to interact with each other so as to prepare the ketazine, wherein the hydrogen peroxide belongs to an industrial product and has the concentration of 25% to 30%; ammonia is added in a form of ammonia water with the concentration of 20% to 25%; and the butanone, the formamide and the inorganic oxide are all in the industrial product level. The method disclosed by the invention can be used for solving the problems that the existing initial coarse products for producing hydrazine hydrate are low in concentration, high in energy consumption, low in efficiency and high in cost and the by-products are difficult to treat, thereby being suitable for the situations in China.
Owner:重庆蓝苗生物科技开发有限公司
Hydrogen peroxide point-of-use wipers
A hydrogen peroxide point of use wetted wiper system includes a packaging with a first, a second, and a third compartment, wherein the separate compartments are mergable. Wipers are disposed in the third compartment. Disposed in the second compartment is dry urea hydrogen peroxide, isolated from the wipers. Disposed in the first compartment is a solvent, wherein the solvent is isolated from the wipers and the urea hydrogen peroxide. The first and second compartments can merge to form a unified compartment such that the solvent is combined with the urea hydrogen peroxide at a point of use to form liquid 6% hydrogen peroxide solution. The unified compartment then merges with the third compartment such that the liquid hydrogen peroxide wets the wipers. The liquid hydrogen peroxide concentration and amount is proportional to the wiper absorptive capacity, number, size, and degree of desired saturation.
Owner:ILLINOIS TOOL WORKS INC
A hollow nanosphere used for loading lipase for vanillin preparation through catalytic oxidation
A hollow nanosphere used for loading lipase for vanillin preparation through catalytic oxidation is disclosed and relates to the technical field of biological catalysis. The method includes (1) preparing a polymer hollow nanosphere; (2) preparing a polymer-TiO2 composite hollow nanosphere; (3) covering the composite hollow nanosphere with a hard shell; and (4) preparing the disclosed hollow nanosphere. The prepared hollow nanosphere is used for loading the lipase. The lipase loading amount in each gram of the hollow nanosphere is 15 mg or above. A prepared lipase catalyst is specially used for vanillin preparation through catalytic oxidation, coniferyl aldehyde is adopted as a raw material, hydrogen peroxide-urea is adopted as an oxygen source of the lipase, the yield of prepared vanillin is 93% or above, and purity of the prepared vanillin is 98% or above.
Owner:MUDANJIANG MEDICAL UNIV
Method for preparing epoxidized fatty acid methyl ester through lipase catalysis
InactiveCN103088082ANot easy to produceMild reaction conditionsFermentationOrganic acidPolymer science
The invention discloses a method for preparing epoxidized fatty acid methyl ester through lipase catalysis. The method comprises the following steps of mixing fatty acid methyl ester, organic acid and lipase uniformly according to a mass ratio of 100g:10-30g:3-5g, then adding 10-20g of urea hydrogen peroxide with mass concentration of 25-50% each time for 3-4 times, importing the reaction product into a separator after reacting for 8-10 hours, standing for layering, and drying to obtain the epoxidized fatty acid methyl ester. The method has the advantages of being mild in reaction conditions and environment-friendly, and the like.
Owner:闭美娥
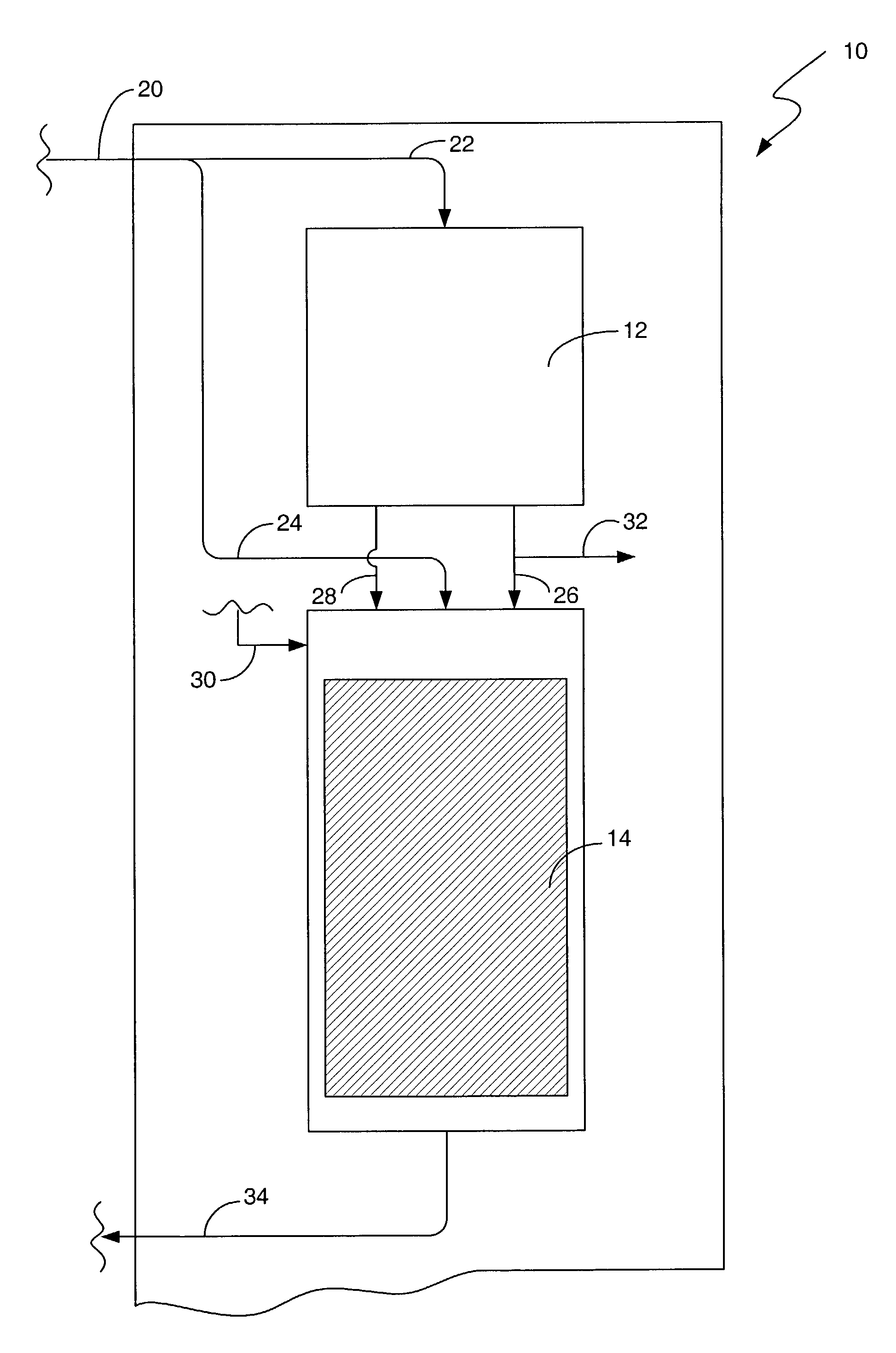
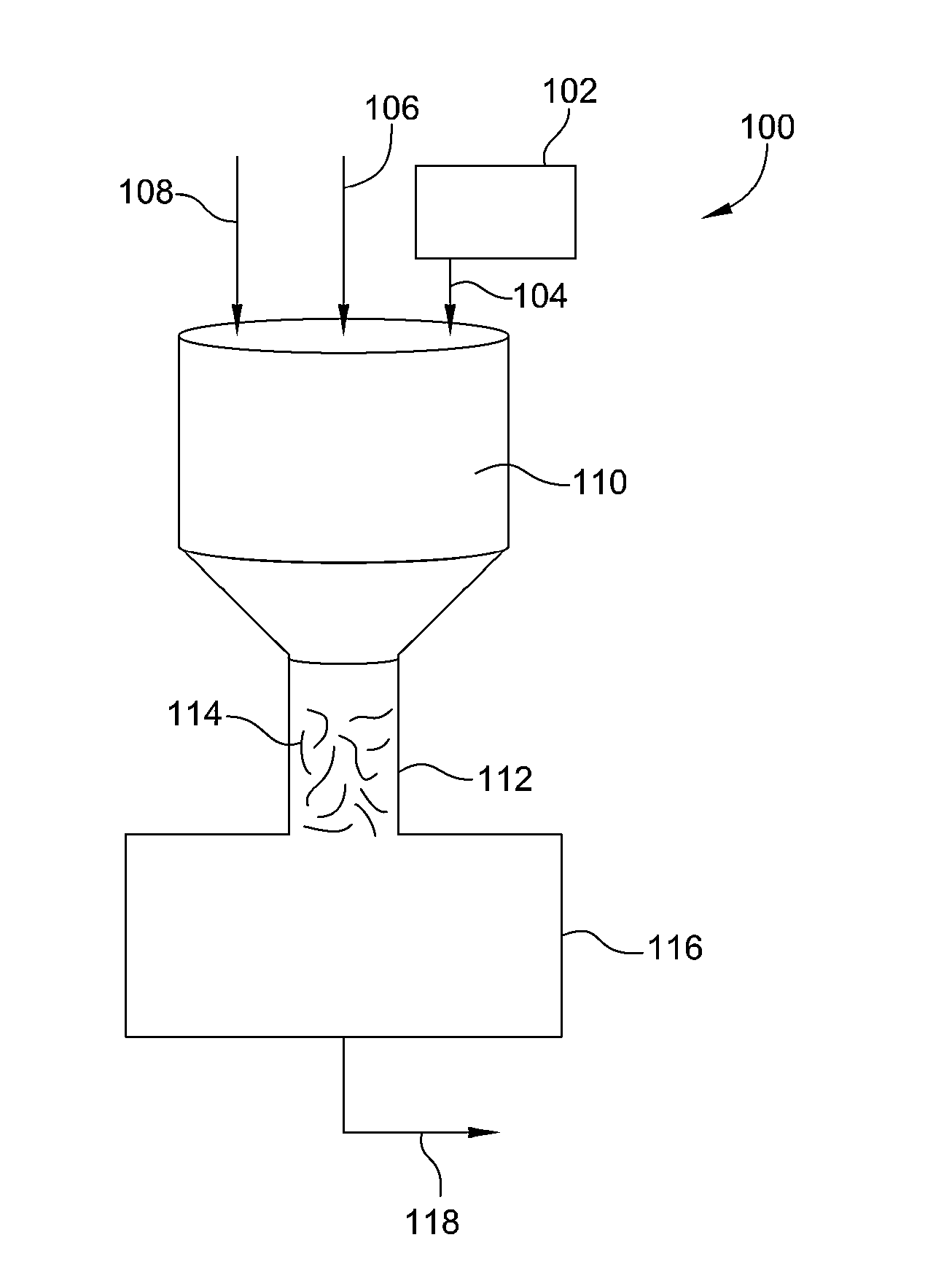
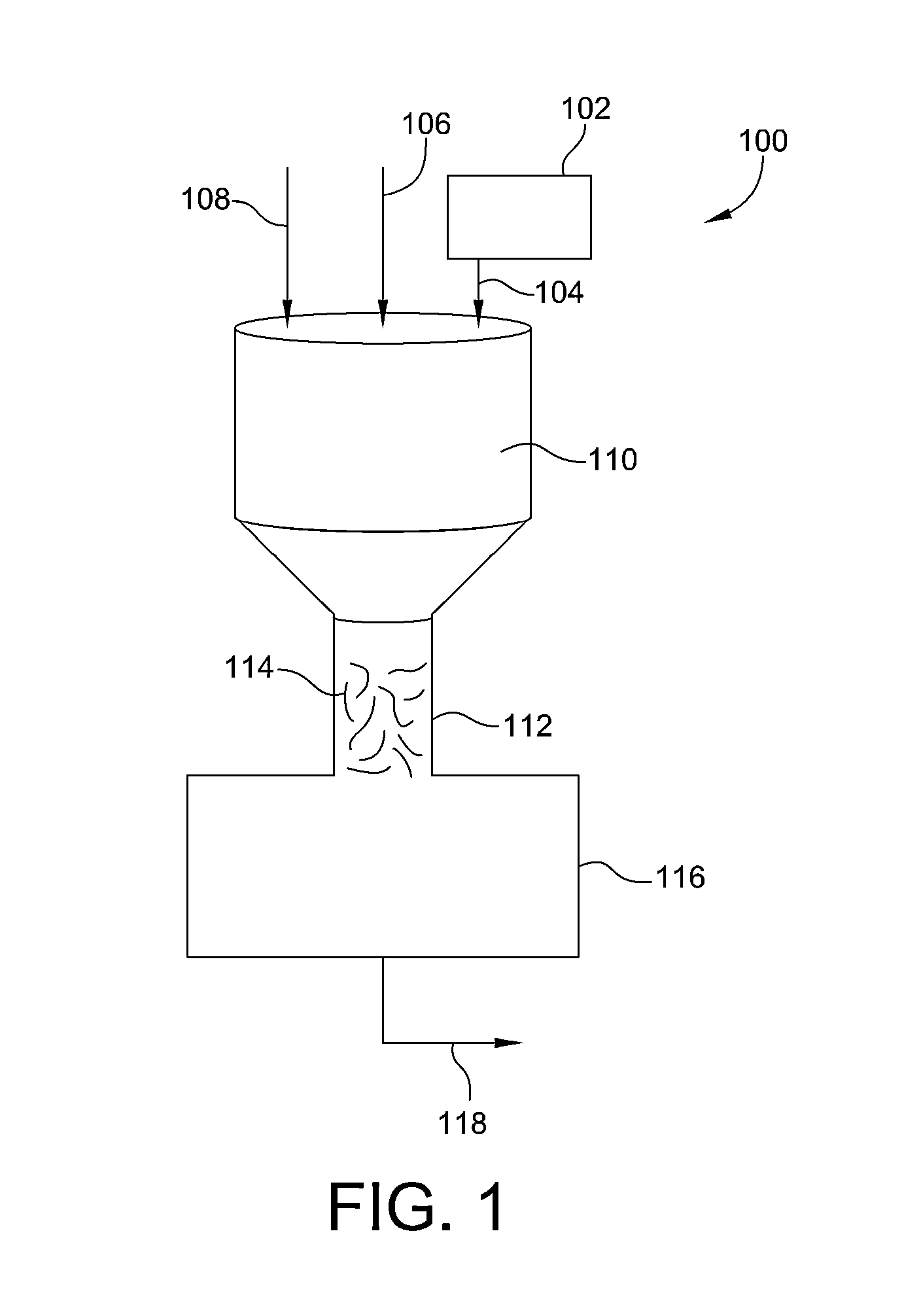
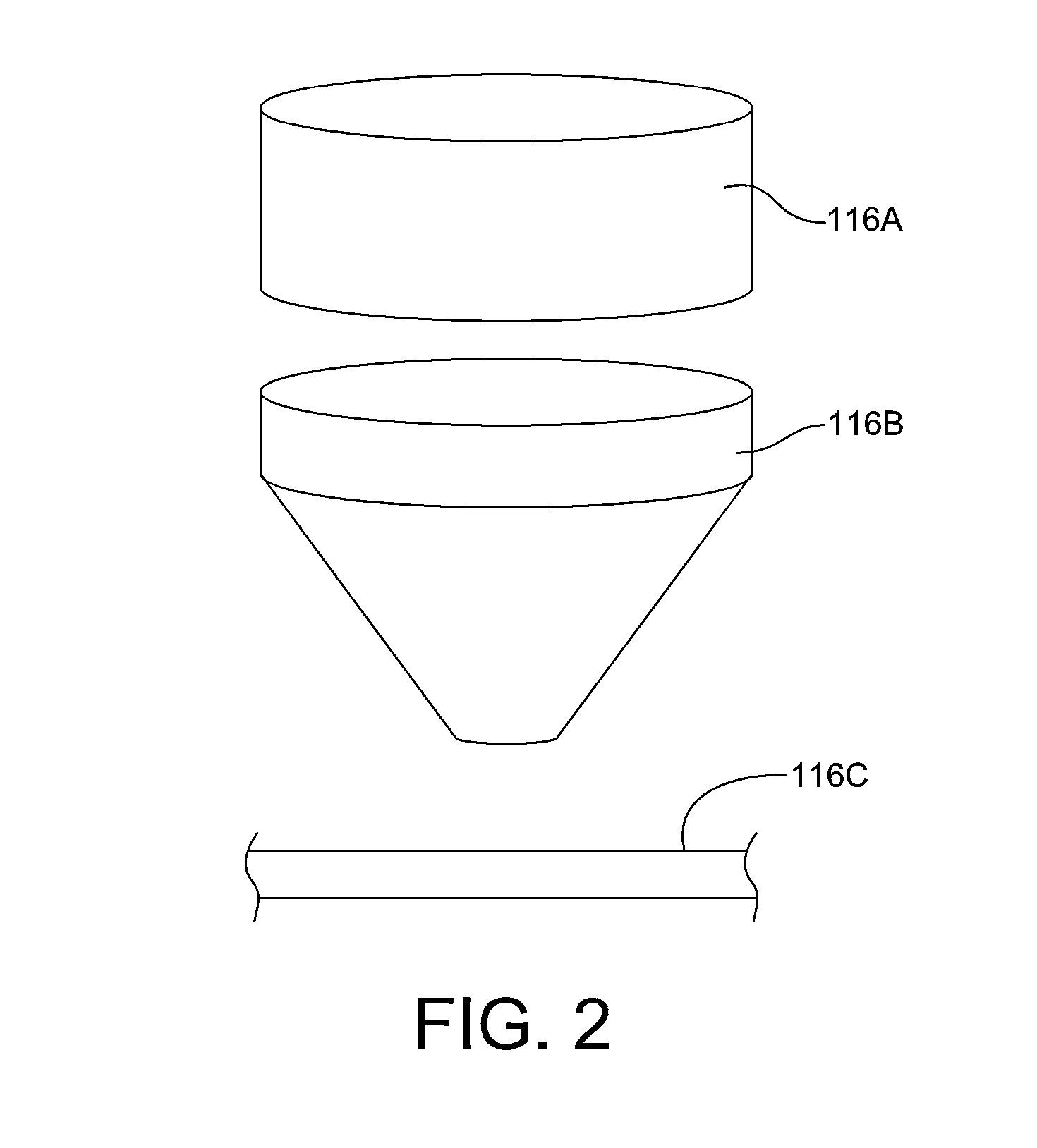
Process and system for removing urea from an aqueous solution
A process for removing urea from an aqueous solution is disclosed herein, the process comprises the steps of: feeding an aqueous solution comprising urea into a mix tank; feeding hydrogen peroxide into the mix tank; feeding at least one soluble catalyst into the mix tank separately from the hydrogen peroxide feed; mixing the aqueous solution comprising urea, hydrogen peroxide, and the at least one soluble catalyst in the mix tank, forming a reactant mixture; and oxidizing the urea in the reactant mixture yielding CO2, N2, and H2O. The soluble catalyst is selected from a group of catalysts that when mixed with the hydrogen peroxide and urea causes the rate of reaction of the oxidation of the urea by hydrogen peroxide to accelerate; such as soluble iron salts. A system configured to carry out the process for removing urea from an aqueous solution is also disclosed herein. The disclosed process and system may be added to or incorporated with existing processes and systems for treating aqueous solutions.
Owner:KLING MILLER LUISA +1
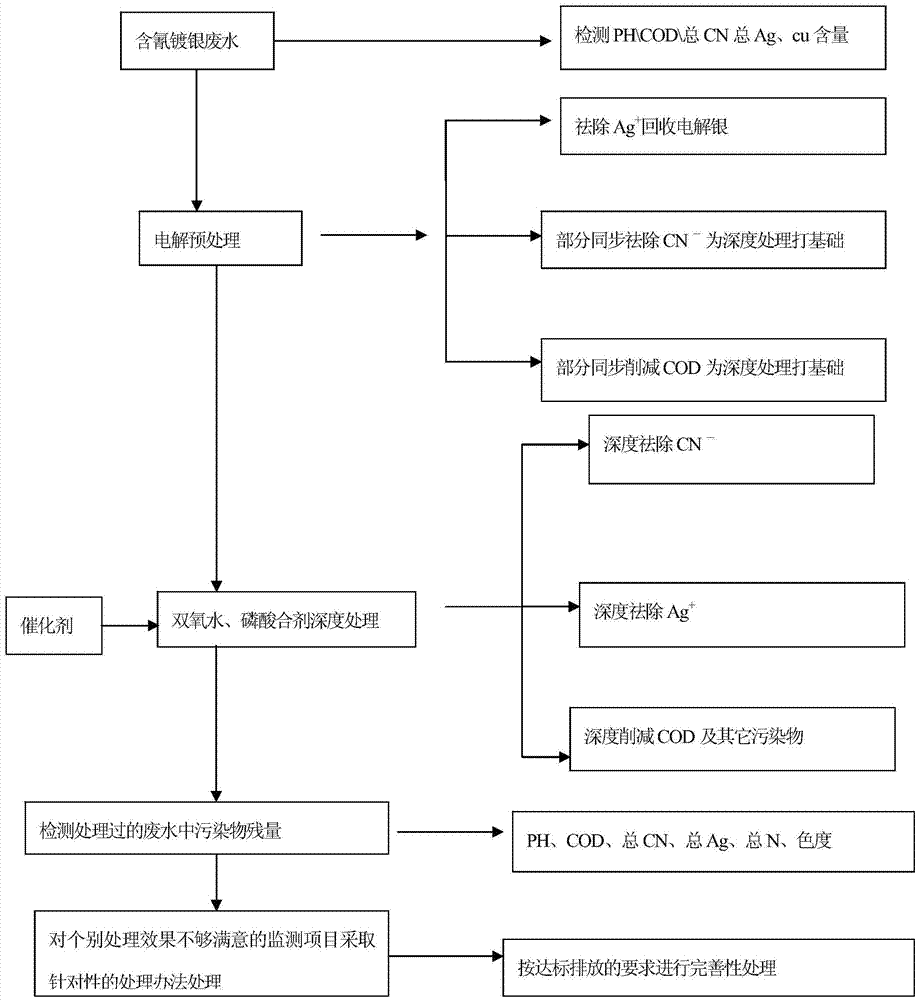
Hydrogen peroxide-phosphoric acid mixture, and preparation method and application thereof
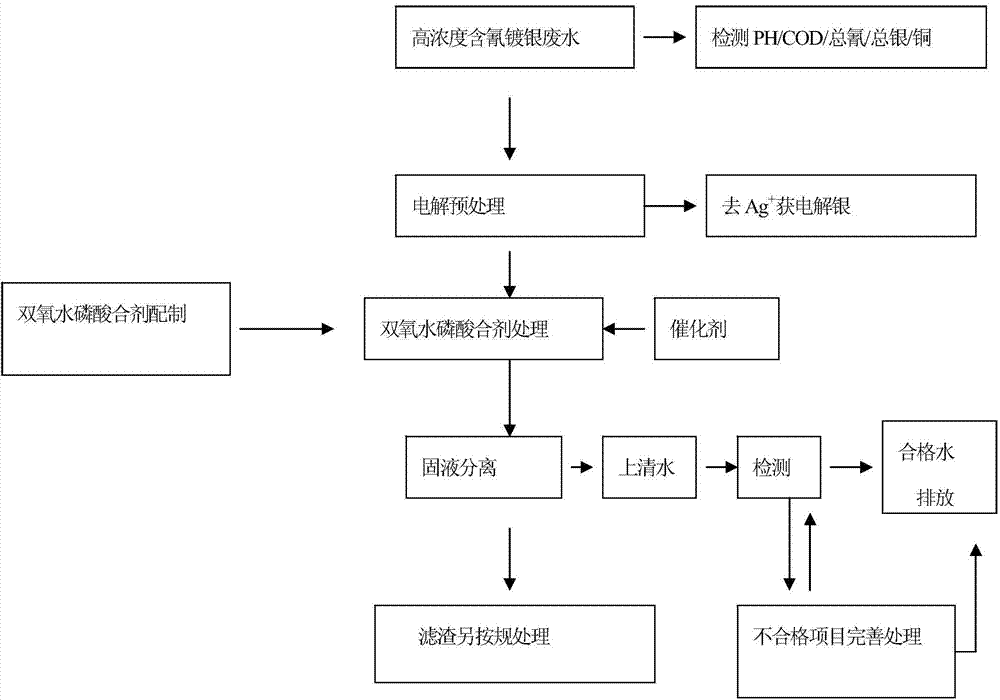
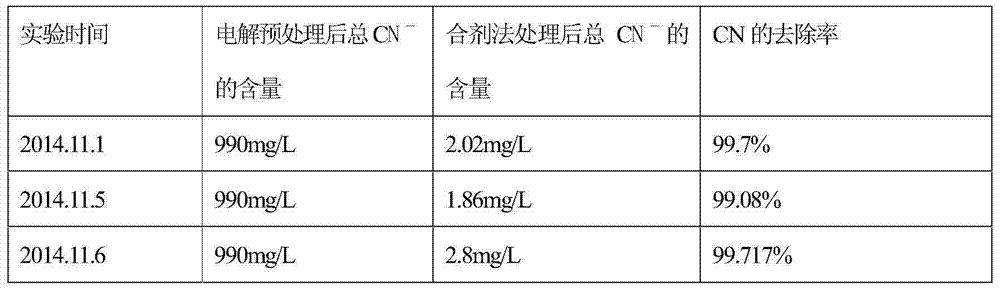
InactiveCN104761042AEliminate pollutionEnsure health and safetyWater contaminantsWaste water treatment from metallurgical processHigh concentrationElectrolysis
The invention provides a hydrogen peroxide-phosphoric acid mixture, and a preparation method and an application thereof. The hydrogen peroxide-phosphoric acid mixture is composed of hydrogen peroxide, phosphoric acid and a stabilizer, a mass ratio of hydrogen peroxide to phosphoric acid is 20:1-50:1, and the stabilizer accounts for 0.1-0.5% of the weight of hydrogen peroxide. The application method of the hydrogen peroxide-phosphoric acid mixture in treatment of cyanogen-containing silvering wastewater comprises the following steps: 1, carrying out electrolyzing pretreatment on the cyanogen-containing silvering wastewater to remove Ag<+> as possible; and 2, treating the pretreated cyanogen-containing silvering wastewater by using the hydrogen peroxide-phosphoric acid mixture. The hydrogen peroxide-phosphoric acid mixture can be used to treat medium and low concentration cyanogen-containing silvering wastewater, and can also be used to treat high concentration cyanogen-containing silvering wastewater. The hydrogen peroxide-phosphoric acid mixture avoids secondary pollution and guarantees the health and safety of operators in the treatment process of the cyanogen-containing silvering wastewater, and allows the precious metal silver to be recovered in the electrolyzing pretreatment process.
Owner:丁建林 +2
Bleaching process and device used in nitric acid preparation process
Owner:宁夏润夏能源化工有限公司
Catalyst for decomposing hydrogen peroxide and application method
ActiveCN106140139AImprove securityEliminate potential hazardsOrganic chemistryMetal/metal-oxides/metal-hydroxide catalystsHydrogen peroxide breakdownDistillation
The invention provides a catalyst for decomposing hydrogen peroxide. The catalyst for decomposing hydrogen peroxide is characterized by being prepared from, by mass, 1%-30% of VI B-group metal oxide, 1%-20% of IV B-group metal oxide and 50%-98% of III A-group metal oxide. According to a method for decomposing hydrogen peroxide in a product of an epoxidation reaction of 3-chloropropene and hydrogen peroxide by adopting the catalyst, most residual hydrogen peroxide in the product of the reaction can be decomposed before distillation separation, potential hazards of the high hydrogen peroxide content in the product of the epoxidation reaction to the follow-up distillation separation process are effectively eliminated, and therefore the safety of producing epoxy chloropropane through the epoxidation reaction of 3-chloropropene and hydrogen peroxide is improved.
Owner:CHINA PETROLEUM & CHEM CORP +1
Process for synthesizing vanillin by adopting bio-enzyme catalytic oxidation method
The invention relates to a method for synthesizing vanillin by adopting a bio-enzyme catalytic oxidation method. The method comprises the following steps: sequentially adding a solvent, a primer coniferyl alcohol / coniferyl aldehyde / ferulic acid, lipase, binary acid or a binary acid derivative and urea hydrogen peroxide in a reaction kettle, wherein lipase accounts for 1-4wt% of the primer isoeugenol, the molar mass of the binary acid or the binary acid derivative is 0.75-1.5 times that of the primer; the molar mass of urea hydrogen peroxide is 1-2 times that of the primer, wherein the adding temperature of urea hydrogen peroxide is 15-35 degrees, the reaction temperature is 20-40 degrees and the reaction time is 14-36 hours; filtering, separating and recycling urea and an enzyme-catalyst, diluting the filtrate by water, extracting by using an organic solvent, recrystallizing by edible alcohol and drying to obtain vanillin; and complexing the mother liquor extracted by the organic solvent by virtue of sodium hydrogen sulfite or sodium sulfite to recover the byproduct of aldehyde. The vanillin yield of the method can reach 85% and the method is simple in reaction step, mild in condition, strong in catalytic specificity, hard to generate byproducts, high in product yield, environmentally friendly and easy to amplify.
Owner:BOTON SHANGHAI BIOLOGICAL TECH CO LTD
Method of removing organic impurities in hydrogen peroxide
ActiveCN103145102AReduce organic contentExtend the life cyclePeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesPurification methodsSolid particle
The invention relates to a hydrogen peroxide purification method in the process of preparing hydrogen peroxide by using an anthraquinone process. The method comprises the following steps: firstly, simultaneously feeding a hydrogen peroxide solution containing organic impurities and a solvent into a mixing device, and completely mixing; feeding the two-phase mixture into a first filter so as to remove solid particles in the two-phase mixture; then, feeding the two-phase mixture into a series-wound coalescer set so as to separate the hydrogen peroxide solution with the solvent containing organic impurities; and finally, feeding the separated hydrogen peroxide solution into a second filter so as to remove trace organic impurities in the hydrogen peroxide solution. In case of treating hydrogen peroxide solutions by using the method disclosed by the invention, the organic matter content of the hydrogen peroxide solutions can be significantly reduced.
Owner:上海睿思化工科技有限公司
Urea hydrogen peroxide disinfectant and preparation method thereof
InactiveCN102206174AEasy to manufactureChange typeUrea derivatives preparationOrganic compound preparationChlorine dioxideDisinfectant
The invention discloses a urea hydrogen peroxide disinfectant and a preparation method thereof. The finished urea hydrogen peroxide disinfectant is prepared by stirring and mixing urine, hydrogen peroxide, trisodium phosphate, salicylic acid and sodium silicate in a certain weight ratio at a certain temperature. The preparation process of the urea hydrogen peroxide disinfectant is simple and convenient; in reaction time, the rise of a reaction temperature can be controlled and the type of the stabilizer can be changed effectively; the crystallization temperature is changed; and the chlorine dioxide can be used for disinfection in various industries such as hospitals, schools, drinking water and the like, the application range is wide and the effect is obvious.
Owner:ANHUI HUANGHUAI VETERINARY MEDICINE
Synthetic method of 2-hydroxy propanedinitrile
InactiveCN102936209ASynthetic cleanSafe and efficient synthesisCarboxylic acid nitrile preparationOrganic compound preparationChemical synthesisAcetic anhydride
The invention relates to a practical synthetic method of 2-hydroxy propanedinitrile based on a chemical synthesis method, mainly solving the problems of conventional synthetic method that the specific operation is complex, the reaction condition is severe, the reaction time is long, the performability is low, and the industrial production cannot be carried out. According to the synthetic method, the conventional 2-(1-hydroxy acetal) propanedinitrile which is easy to obtain is adopted and reacts with peroxy acid to generate the 2-hydroxy propanedinitrile, wherein the peroxy acid is prepared by acetic anhydride or trifluoroacetic anhydride and urea hydrogen peroxide. The chemical synthetic method of 2-hydroxy propanedinitrile provided by the invention is brand new, short in reaction time, low in cost, and suitable for industrial scale production.
Owner:SHANGHAI STA PHARMA R&D CO LTD
Aflatoxin enzyme linked immunosorbent assay kit and detecting method
The invention discloses an aflatoxin enzyme linked immunosorbent assay kit and a detecting method. The aflatoxin enzyme linked immunosorbent assay kit comprises: an enzyme label plate, an aflatoxin B1standard solution, an aflatoxin B1 antibody working solution, an enzyme labeled second antibody working solution, a substrate solution A, a substrate solution B, a stop solution, a concentrated diluent and a concentrated cleaning solution. The substrate solution A is a citric acid-dibasic sodium phosphate buffer solution containing 0.5 mmol / L of hydrogen peroxide urine. The substrate solution B is an ethanol solution of tetramethylbenzidine. The stop solution is 2 mol / L of a sulfuric acid aqueous solution. The enzyme label plate is coated with an aflatoxin B1 coating antigen. The aflatoxin B1coating antigen is obtained by coupling the aflatoxin B1 antigen with bovine serum albumin. Compared with a traditional detecting method, the above detecting method of the kit is relatively simple inboth detecting process and sample processing process, and the detecting process and the operation are both relatively convenient.
Owner:CHINA NAT CENT FOR FOOD SAFETY RISK ASSESSMENT +1
Preparation method of vinpocetine analogue
A preparation method of a vinpocetine analogue comprises the following steps: using UHP (urea hydrogen peroxide) for selective oxidation of a tabersonine analogue, then performing reduction, rearrangement, crystallization, and the like; or using the UHP for selective oxidation of a tabersonine reduction analogue (vinca-fuming analogue), then performing rearrangement, crystallization, and the like to obtain the vinpocetine analogue. Compared with the prior art, the preparation method has the advantages of high yield, direct quantification, simple operation, mild reaction conditions, suitability for industrial production, the yield reaching over 87%, more than 99% of HPLC (high performance liquid chromatography), a white product, good quality and low preparation cost.
Owner:HUNAN KEYUAN BIO PRODS
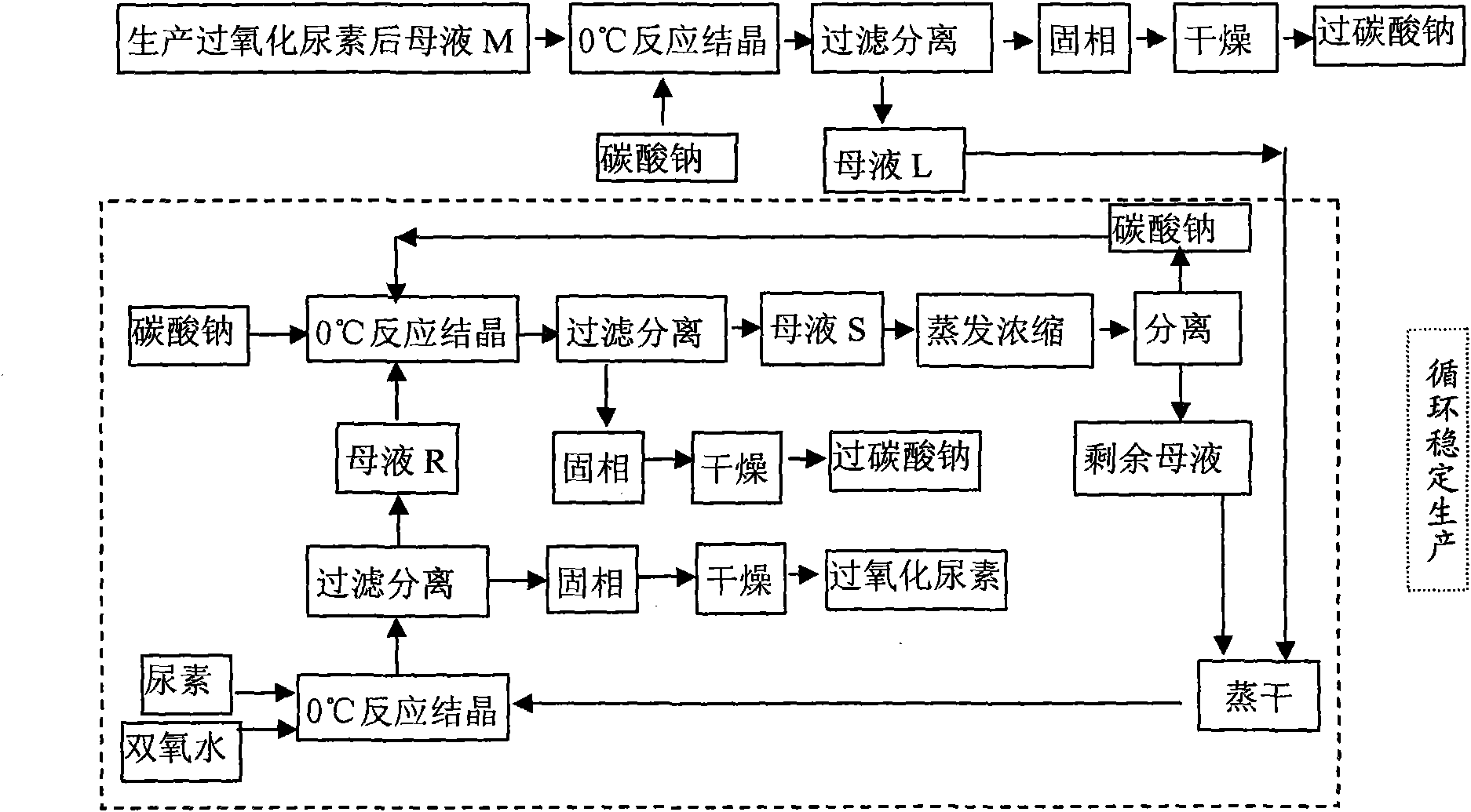
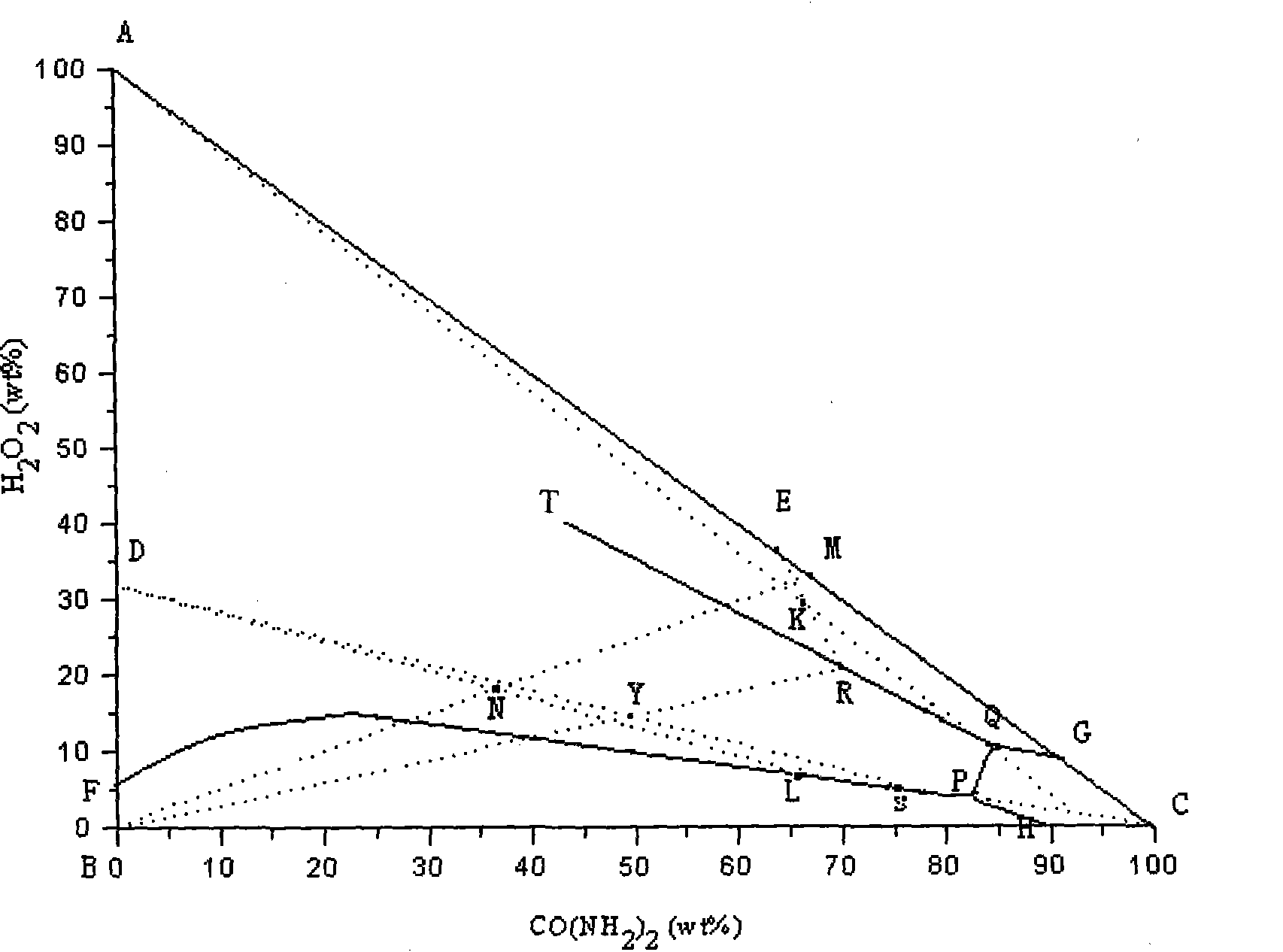
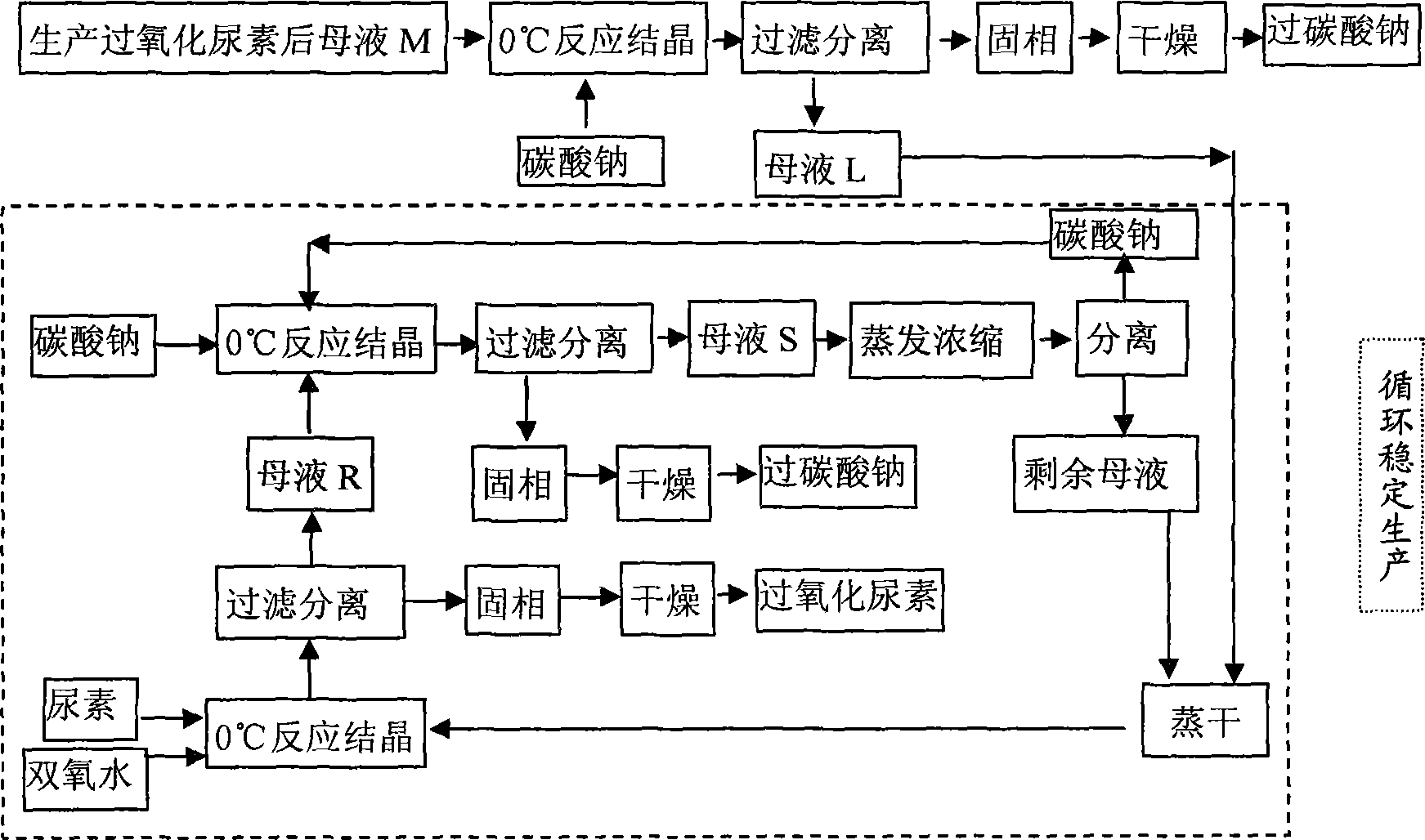
Method of joint production of urea hydrogen peroxide and sodium percarbonate
InactiveCN101786969AReduce adverse effectsReduce manufacturing costUrea derivatives preparationOrganic compound preparationUrea hydrogen peroxideAdverse effect
The invention discloses a method of joint production of urea hydrogen peroxide and sodium percarbonate, comprising the following steps: in a material system at the following mass ratio that sodium carbonate: urea: hydrogen peroxide: water is 1:1.30-1.60:0.42-0.45:2.75-3.20, crystallization filtering is carried out at the temperature of 0 DEG C, the obtained solid phase is dried at the temperatureof 60 DEG C to obtain sodium percarbonate, and the residual mother solution S is evaporated and concentrated; and after parts of separated sodium carbonate are evaporated to dryness, urea and hydrogen peroxide of which the mass concentration is 30% are added to ensure that the mass ratio in the system is as follows: sodium carbonate: urea: hydrogen peroxide: water is 1:14.00-16.00:5.50-7.50:14.00-16.50, crystallization filtering is carried out at the temperature of 0 DEG C, the obtained solid phase is urea hydrogen peroxide, and sodium carbonate is added into the liquid phase to ensure that the mass ratio in the system is still as follows: sodium carbonate: urea: hydrogen peroxide: water is 1:1.30-1.60:0.42-0.45:2.75-3.20, and thus cycle production is realized. The invention adopts mild reaction conditions and convenient operation, improves the economy of the urea hydrogen peroxide preparation technology, avoids adverse effect on environment due to mother solution abandonment and lowers production cost.
Owner:HEBEI UNIV OF TECH
Method for synchronously synthesizing hydrogen peroxide and peroxyacetic acid
ActiveCN102616751AThe synthesis process is simpleReduce contentOrganic compound preparationPeroxides/peroxyhydrates/peroxyacids/superoxides/ozonidesChemical synthesisDisinfectant
The invention discloses a method for synchronously synthesizing hydrogen peroxide and peroxyacetic acid, wherein, hydrogen and oxygen are taken as gas sources, alcohol, water and acetic acid are taken as reaction solvent, and phosphoric acid and salt or acid which can generate Br- after being dissolved are taken as reaction assistants; and hydrogen peroxide and peroxyacetic acid can be synthesized synchronously under the conditions that the pressure ranges from 1 to 20 MPa and the temperature ranges from minus 20 to 15 DEG C. The mixed solution that is prepared according to the method can be used as raw material for preparing compound disinfectant and bleaching agents and can also be used as intermediates for other chemical synthesis.
Owner:LIMING RES INST OF CHEM IND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com