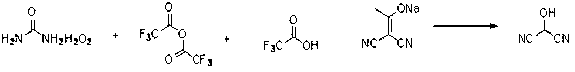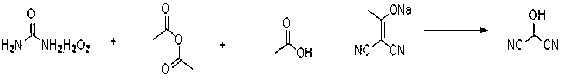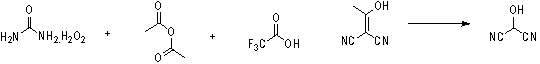Synthetic method of 2-hydroxy propanedinitrile
A technology of hydroxypropanedicyanide and a synthesis method, which is applied in chemical instruments and methods, preparation of organic compounds, organic chemistry, etc., can solve the problems of short reaction time, low cost, harsh reaction conditions, etc., and achieves short reaction time and cost. Low, simple post-processing effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Trifluoroacetic anhydride (14mL, 0.1mol) was added dropwise to urea hydrogen peroxide (9.4g, 0.1mol) and dichloromethane (100 mL) at 0°C, and then stirred at room temperature for 3 hours to obtain trifluoroperoxy acetic acid. Dissolve 2-(1-hydroxyacetal) propanedicyanide sodium salt (13g, 0.1mol) in 150 mL water, add trifluoroacetic acid (7.79 mL, 0.2mol) at 0°C, and then add the peroxy Trifluoroacetic acid was added dropwise, stirred at 35°C for 2 hours, and the reaction was completed by TLC analysis. The reaction solution was quenched with a 2 mol / liter sodium sulfite aqueous solution, the reaction solution was extracted with ethyl acetate, separated, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum at low temperature to obtain an oily liquid product 2-hydroxypropanedicyanide (6.6g, 80 %). 1 HNMR (400 MHz, CD 3 OD): d 5.74 (s, 1H).
Embodiment 2
[0018]
[0019] Acetic anhydride (9 mL, 0.1 mol) was added dropwise to urea hydrogen peroxide (9.4 g, 0.1 mol) and dichloromethane (100 mL) at 0°C, and then stirred at room temperature for 1 hour to obtain peracetic acid. Dissolve 2-(1-hydroxyacetal)propanedicyanide sodium salt (13g, 0.1mol) in 150 mL water, add acetic acid (28.8 mL, 0.5mol) at 0°C, and then drop the previously prepared peracetic acid Add it in, stir at 25°C for 4 hours, and the reaction is complete as detected by thin-layer analysis. The reaction solution was quenched with 2 mol / L sodium sulfite aqueous solution, the reaction solution was extracted with ethyl acetate, separated, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum at low temperature to obtain the oily liquid product 2-hydroxypropanedicyanide (6.1 g, 74 %). 1 HNMR (400 MHz, CD 3 OD): d 5.74 (s, 1H).
Embodiment 3
[0021]
[0022] Trifluoroacetic anhydride (14mL, 0.1mol) was added dropwise to urea hydrogen peroxide (9.4g, 0.1mol) and dichloromethane (100mL) at 0°C, then stirred at room temperature for 2 hours to obtain trifluoroperoxy acetic acid. Dissolve 2-(1-hydroxyacetal) propanedicyanide (10.8g, 0.1mol) in 150 mL of water, add acetic acid (11.52 mL, 0.2mol) at 0°C, and then add peroxytrifluoroacetic acid prepared above Add it dropwise, and stir at 25°C for 6 hours. TLC analysis detects that the reaction is complete. The reaction solution was quenched with 2 moles / liter of sodium sulfite aqueous solution, the reaction solution was extracted with ethyl acetate, separated, dried over anhydrous sodium sulfate, filtered and concentrated in vacuum at low temperature to obtain the oily liquid product 2-hydroxypropanedicyanide (7g, 85% ). 1 HNMR (400 MHz, CD 3 OD): d 5.74 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com