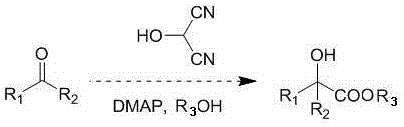
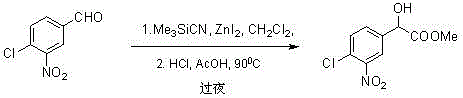Method for synthesizing a-hydroxycarboxylate from 2-hydroxypropanedicyanide
A technology of hydroxymalonocyanide and hydroxycarboxylate, which is applied in the preparation of carboxylic acid esters, chemical instruments and methods, and the preparation of organic compounds, and can solve problems such as high cost, complicated operation and post-processing, and long reaction time. To achieve the effect of short reaction time, clean reaction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015]
[0016] Benzaldehyde (106 mg, 1 mmol) was dissolved in 2 mL of methanol, acetic acid solution of 2-hydroxypropanedicyanide (1 mL, 1 mol / L) was added, and then 4-dimethylaminopyridine (12 mg) was added rapidly, and stirred at 0°C 10 minutes. The reaction solution was concentrated and separated by column chromatography to obtain methyl α-hydroxyphenylacetate (151 mg, 91%). 1 H NMR (400 MHz, CD 3 OD) δ7.36-7.29 (m, 5H), 5.17 (s, 1H), 3.67 (s, 3H).
Embodiment 2
[0018]
[0019] 3,4-Dimethoxybenzaldehyde (166 mg, 1 mmol) was dissolved in 2 mL of methanol, and 2-hydroxypropanedicyanide in trifluoroacetic acid (0.4 mL, 5mol / L) was added, followed by the rapid addition of 4-dimethoxy Aminopyridine (12mg), stirred at room temperature for 40 minutes. The reaction solution was concentrated and separated by column chromatography to obtain methyl α-hydroxy-3,4-dimethoxyphenylacetate (199 mg, 88%). 1 H NMR (400 MHz, CD 3 OD) δ 7.03 (d, J=1.6 Hz, 1H), 7.95-7.92 (m, 2H), 5.11 (s, 1H), 3.82 (s, 3H), 3.81 (s, 3H), 3.68 (s, 3H ).
Embodiment 3
[0021]
[0022] 2-Formaldehyde furan (96 mg, 1 mmol) was dissolved in 2 mL of methanol, and 2-hydroxypropanedicyanide in trifluoroacetic acid solution (0.2 mL, 10mol / L) was added, and then 4-dimethylaminopyridine (12 mg) was added rapidly , stirred at room temperature for 100 minutes. The reaction solution was concentrated and separated by column chromatography to obtain methyl α-hydroxy-2-furanacetate (137 mg, 86%). 1 H NMR (400MHz, CD 3 OD) δ 7.46-7.45 (m, 1H), 6.38-6.37 (m, 2H), 5.21 (s, 1H), 3.73 (s,3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com